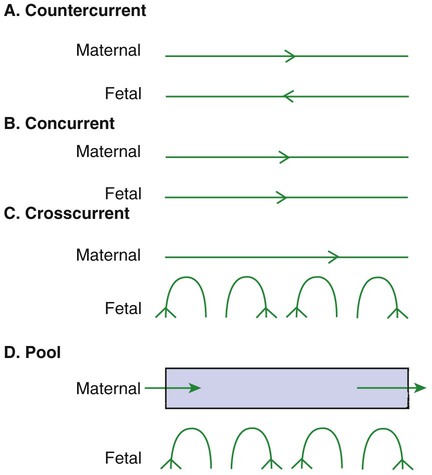
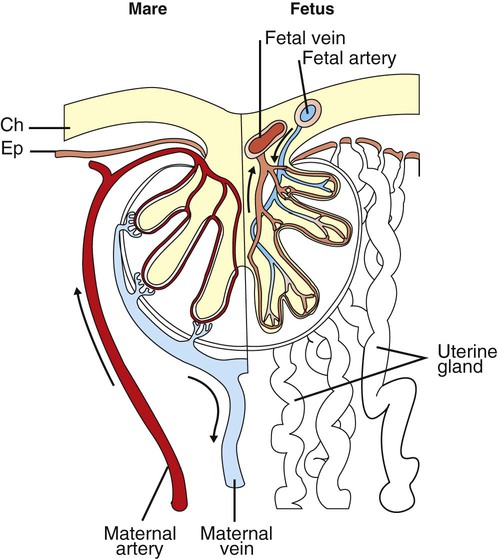
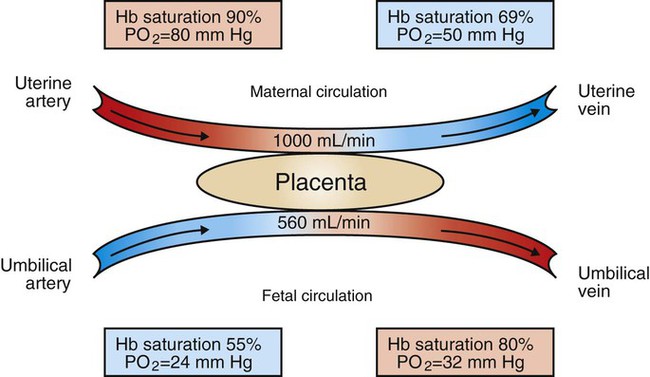
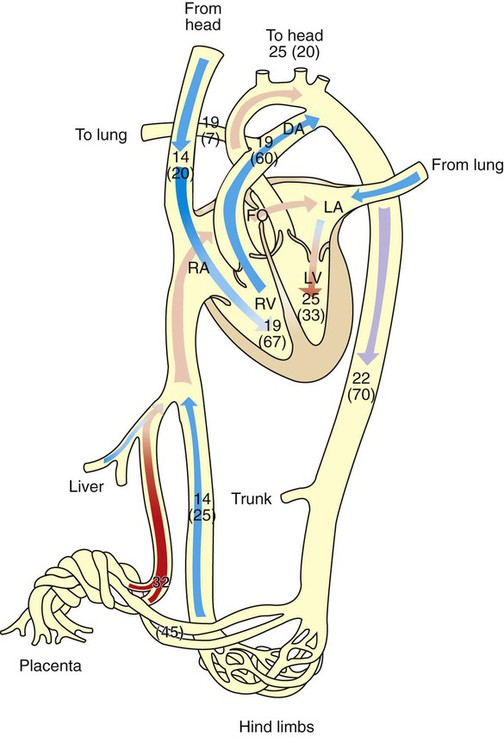
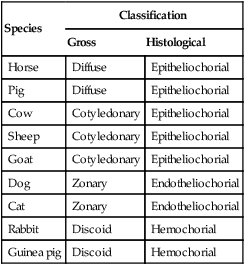
1. The fetus depends on the placenta for the exchange of gas, nutrients, and metabolic byproducts. 2. The efficiency of gas exchange at the placenta depends on the species-variable arrangement of fetal and maternal blood vessels. 3. The fetal circulation mixes oxygenated and deoxygenated blood at several points, so the fetus exists in a state of hypoxemia. 4. Fetal oxygen transport is assisted by fetal hemoglobin, which has a high affinity for oxygen. 5. The lung develops in three stages, and pulmonary surfactant must be present at birth. 6. At or shortly after birth, umbilical vessels rupture, pulmonary vascular resistance decreases, and the foramen ovale and ductus arteriosus close. The gross appearance of the placenta of different species varies widely. In horses and pigs the placenta is diffuse and covers most of the uterine epithelium. In ruminants the placenta has rows of discrete circular-to-oval cotyledons that are attached to approximately 100 highly vascularized caruncles in the uterine epithelium. In dogs the placenta is zonary, forming a circular band around the allantochorion of the puppy. Table 51-1 lists types of placentation for different species. TABLE 51-1 Placentation in Domestic Mammals In addition to differing in the amount of uterine surface to which they are attached, placentas also differ in the number of layers of cells that separate the maternal and fetal blood (see Table 51-1). In horses, pigs, sheep, and cows the fetal chorion is applied to the maternal uterine epithelium (epitheliochorial placentation), whereas in cats and dogs the chorion is applied to the endothelium of maternal vessels (endotheliochorial placentation); in rodents and most primates the chorion invades the uterine mucosa and erodes the maternal capillaries, so it becomes bathed by maternal blood (hemochorial placentation). The exchange of gases and other substances across the placenta is determined by several factors, including the amount of surface apposition between fetal and maternal tissues and the number of layers of cells separating fetal and maternal blood. However, a major factor determining exchange is the arrangement of fetal and maternal blood vessels within the small, interdigitating villi of the placenta (Figure 51-1). Countercurrent flow of maternal and fetal blood provides the most efficient exchange and allows equilibration of fetal and maternal arterial gas tensions. Concurrent flow of fetal and maternal blood allows fetal vessels to equilibrate with the maternal venous gas tensions. In crosscurrent and pool types of equilibrators, fetal capillaries loop down to maternal vessels or into a pool of maternal blood. No simple model easily describes these types of exchangers. It is likely that several different arrangements of vessels are found in the placentas of all species, but some seem to have more of the characteristics of countercurrent exchangers, and others have those of venous equilibrators. Figure 51-2 shows the arrangement of vessels in the microcotyledon of the horse, a species in which fetal and maternal blood flow is primarily countercurrent. The cotyledonary placenta of sheep functions as a venous equilibrator, whereas the hemochorial placenta of the rabbit seems to be a countercurrent exchanger. Placental gas exchange has been best studied in the sheep (Figure 51-3). Maternal blood enters the uterus through the uterine artery with an oxygen tension (PO2) of 80 mm Hg and leaves through the uterine vein with a PO2 of 50 mm Hg. Some of the blood entering the uterus supplies the myometrium and endometrium, but most participates in gas exchange in the cotyledon. Fetal arterial blood reaches the placenta through the umbilical artery and enters the cotyledon with a PO2 of 24 mm Hg. Placental gas exchange occurs, and the blood leaving the placenta in the umbilical veins has a PO2 of only 32 mm Hg. This is because the sheep placenta is a venous equilibrator, so the maximal possible PO2 would be 50 mm Hg. However, this maximum is not reached because venous blood, which has provided nutrient blood flow to the chorion, dilutes the better-oxygenated blood draining from the cotyledon. The countercurrent exchanger of the horse is apparently more efficient because umbilical venous PO2 averages 48 mm Hg. In the adult the cardiac output of the right and left ventricles is separate and perfuses the pulmonary and systemic circulations, respectively. In the fetus the output of the two sides of the heart mixes at several points, so it is convenient to use the term cardiac output to refer to the combined output of the right and left ventricles. The combined cardiac output averages 500 mL/min/kg in fetal sheep; the output of the right ventricle exceeds that of the left (Figure 51-4). Because the right and left ventricles of the fetus pump into a common circulation, the two are of equal size and wall thickness.
Fetal and Neonatal Oxygen Transport
The Fetus Depends on the Placenta for the Exchange of Gas, Nutrients, and Metabolic Byproducts
Species
Classification
Gross
Histological
Horse
Diffuse
Epitheliochorial
Pig
Diffuse
Epitheliochorial
Cow
Cotyledonary
Epitheliochorial
Sheep
Cotyledonary
Epitheliochorial
Goat
Cotyledonary
Epitheliochorial
Dog
Zonary
Endotheliochorial
Cat
Zonary
Endotheliochorial
Rabbit
Discoid
Hemochorial
Guinea pig
Discoid
Hemochorial
The Efficiency of Gas Exchange at the Placenta Depends on the Species-Variable Arrangement of Fetal and Maternal Blood Vessels
The Fetal Circulation Mixes Oxygenated and Deoxygenated Blood at Several Points, So the Fetus Exists in a State of Hypoxemia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree