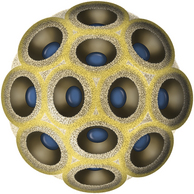
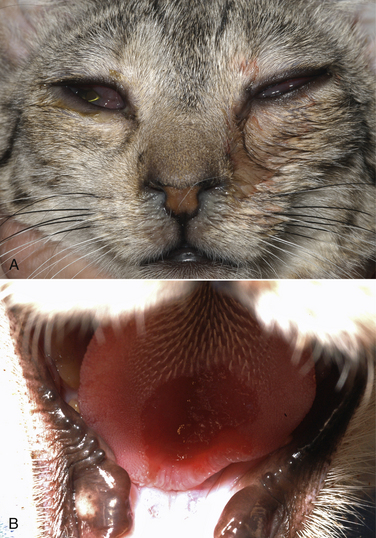
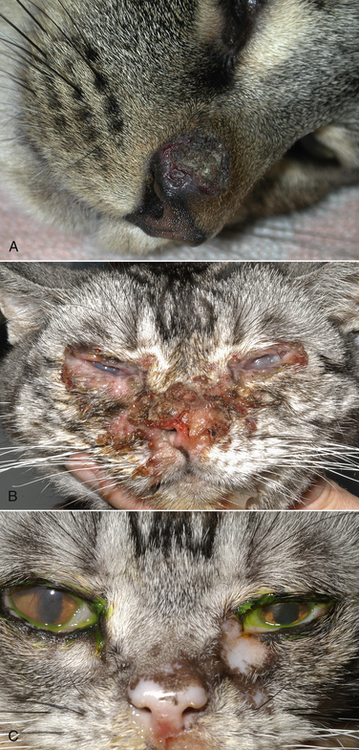
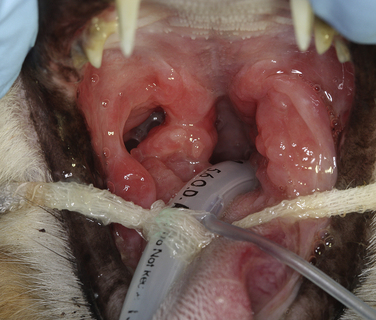
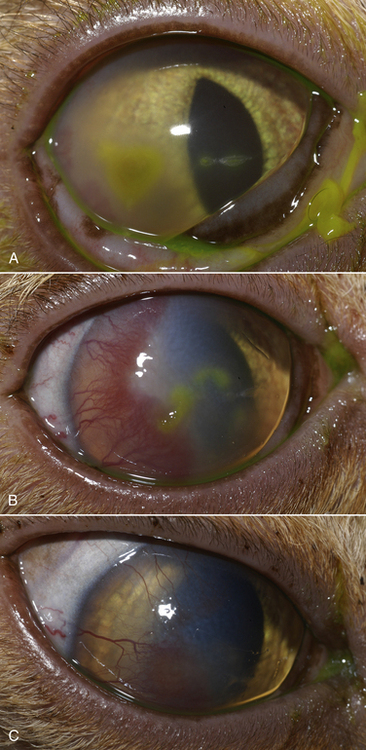
Chapter 23 Infectious feline upper respiratory tract disease (URTD) is a widespread and important cause of morbidity and mortality where large numbers of cats are housed together, especially in overcrowded or stressful conditions.5 Multiple pathogens are involved and co-infections are common (Table 23-1). The most prevalent viral causes of URTD are feline herpesvirus-1 (FHV-1) and feline calicivirus (FCV). Influenza viruses also cause respiratory disease in cats but are relatively rarely identified. Bacterial causes of respiratory disease in cats include Bordetella bronchiseptica, Chlamydia felis, and Mycoplasma species. Streptococcus canis and Streptococcus equi subspecies zooepidemicus can also play a role in shelter situations and catteries. More information on the bacterial causes of feline URTD can be found in Chapters 33, 34, 38, and 40. TABLE 23-1 Viral Causes of Transmissible Respiratory Disease in Cats ∗Incubation and shedding periods are approximate and may differ when co-infections or immune suppression operate. FHV-1 is a large, enveloped DNA virus that has a worldwide distribution. Most cats are exposed to FHV-1 during their lifetime. FHV-1 is an alphaherpesvirus that is closely related to canine herpesvirus-1, and to a lesser extent, herpes simplex virus. There is only a single serotype of FHV-1. Isolates are also genetically similar, yet some variation in strain virulence exists.6 Using culture, FHV-1 has been detected in 0% to 39% of cats with URTD, although in some catteries and shelters with endemic FHV-1 infection, the prevalence may be much higher. When sensitive PCR assays are used to detect FHV-1, prevalences of infection that approach 100% have been detected in some groups of cats with acute URTD.7 The prevalence of shedding by apparently healthy cats has ranged from 0% to 10% and most often has been lower than 2%.5,8–14 Virtually all infected cats develop latent infection, which primarily occurs in the trigeminal ganglia. FHV-1 DNA can also be detected in other tissues of the head, such as the cornea and nasal cavity, but whether this is a true state of latency or just chronic persistent infection is not clear. Reactivation of shedding, with or without concurrent clinical signs of URTD, occurs in less than half of latently infected cats 4 to 12 days after stress.15 Examples of stressors include transportation (such as to a veterinary clinic, boarding or breeding facility, shelter, or cat show), lactation, exposure to new cats, concurrent illness, or treatment with immunosuppressive drugs such as glucocorticoids. The duration of shedding after reactivation ranges from 1 to 13 days (mean, 7 days).16,17 Shedding that coincides with lactation results in infection of susceptible kittens. FHV-1 survives a maximum of 18 hours at room temperature and is readily inactivated by drying and most disinfectants. Because of this, transmission occurs primarily through close contact, although fomites remain a very important mode of transmission in crowded environments. Aerosol transmission is of lesser importance.15,18 Aerosols generated by sneezing cats typically travel no further than a distance of 4 to 5 feet. Feline calicivirus (FCV) is a non-enveloped, single-stranded RNA virus with a spherical capsid that is studded with cup-shaped depressions (calici = “cup”) (Figure 23-1). Like FHV-1, FCV commonly causes feline URTD, accounting for 10% to over 50% of cases. The highest prevalences occur in large multicat environments. Natural infection of dogs by FCV-like strains has been reported.19 Like other RNA viruses, the genome of FCV continually undergoes rapid mutation, which increases the diversity of strains over time. Although considerable antigenic diversity exists among FCV isolates, the degree of antigenic cross-reactivity between them is sufficient for them to be classified as a single serotype. Nucleotide sequence analysis also suggests that isolates worldwide are a single, but highly diverse, group. No correlations have been found between the genetic composition of FCV strains, different clinical manifestations of disease, or geographical location. Infected cats can develop a persistent oropharyngeal infection (>1 month in duration) in the absence of obvious clinical signs. This is termed the carrier state for FCV, and may result from immune evasion through antigenic variation of the capsid protein.20 FCV is shed continuously from the oropharynx, and the magnitude of shedding varies with time and between individual cats.21 Carrier cats serve as a source of infection for other cats. In many cats, shedding terminates weeks to months after infection, but in a few cats, shedding is lifelong. A single cat can be simultaneously infected with multiple variants of FCV, each derived from the original infecting strain as a result of genetic mutation, drift, and selection pressures.20 Because of the chronic carrier state, the prevalence of FCV infection in healthy cats is high and ranged from 8% of household cats to 24% of show cats.12 Environmental persistence of FCV is considerably more prolonged than that of FHV-1, and FCV resists routine disinfection with quaternary ammonium compounds. Susceptibility to disinfectants may vary between FCV strains.22 Survival in the environment has been demonstrated for as long as 28 days,23 and related caliciviruses persist in a dried state for several months.24 As a result, fomites are a very important means of transmission. FCV is also transmitted through direct contact with respiratory secretions and through aerosols. Fleas may spread FCV through their feces or when cats ingest fleas while grooming.25 Highly virulent FCV strains have been isolated from outbreaks of severe systemic febrile illness in cats in the United States and Europe known as virulent systemic disease (VSD),26–31 which was first described in California in 1998. Shelter cats that were hospitalized in veterinary clinics have been a source of infection in many outbreaks, and for each outbreak, the FCV strain involved has differed. VSD has also been described in one cat from a multiple-cat household, that is, in the absence of an outbreak.32 Otherwise healthy, adult, vaccinated cats are often affected, whereas kittens tend to show less severe signs. Although infections typically spread rapidly in outbreaks, including through fomites to pet cats of hospital staff, spread of disease has been limited to affected clinics or shelters, with no further spread within the community, and outbreaks resolve within approximately 2 months once appropriate control measures are instituted. Pandemic H1N1 influenza viruses originated from pigs and have caused widespread illness in humans. Natural infection with these strains has been reported in cats from the United States and Europe. Affected cats had signs of fever and respiratory and gastrointestinal tract disease and, in some cases, death.4,33–35 Transmission from sick humans who were in contact with the cats was suspected. Cat-to-cat transmission may also have occurred.34 Highly pathogenic avian origin H5N1 influenza virus infection, which emerged as a cause of human illness in Hong Kong in 1997 and subsequently spread worldwide,36 has been detected in sick and apparently healthy domestic cats and wild felids from southeast Asia3,37,38 and central Europe.39,40 Nevertheless, no evidence of infection was found in more than 170 cats where infected birds had been identified in Germany and Austria.41 Infection of cats follows direct or indirect contact with infected birds, especially consumption of raw poultry; cat-to-cat transmission may also occur.37 Canine H3N2 virus infection was associated with bronchopneumonia in shelter-housed cats in China and Korea.42 Experimentally, cats can also be infected with other influenza viruses, including human H2N2 and H3N2, and avian origin H7N7 and H7N3 viruses.43,44 Widespread evidence of exposure to pandemic H1N1, seasonal H1N1, and seasonal H3N2 was found in 400 cats from Ohio in the United States.45 Seropositive cats were 7.4 times more likely to have had respiratory illness than seronegative cats, and twice as likely to have had nonrespiratory illness than seronegative cats. More information on influenza viruses can be found in Chapter 17. After direct or indirect contact with virus in respiratory secretions from the conjunctiva, nasal cavity and oropharynx, FCV and FHV-1 replicate within lymphoid and epithelial cells in the upper respiratory tract and cause cytolysis. FHV-1 also replicates in corneal epithelial cells.46 Although FHV-1 prefers to replicate in the cooler tissues of the upper respiratory tract, systemic infection with viremia occurs in some cats infected with FHV-1 and may be more likely to occur in neonates and debilitated cats. FCV and influenza viruses also replicate systemically. FCV is shed in the urine and feces of infected cats, in addition to respiratory secretions. Influenza viruses are shed in both respiratory secretions and the feces. Avian origin H5N1 influenza viruses replicate initially in the lower respiratory tract (type II pneumocytes and alveolar macrophages) or gastrointestinal tract (for example, after ingestion of an infected bird), which is followed by severe systemic infection with necrosis and inflammation in multiple organs.38 In contrast, severe systemic infection is not a feature of H1N1 influenza virus infections in cats.47 Clinical signs of viral URTD occur after an incubation period of 2 to 6 days, although longer incubation periods are also possible, and incubation periods as short as 1 to 2 days can occur in cats infected with influenza viruses.47 Viral shedding occurs as early as 24 hours after infection, and often before the onset of clinical signs. Clinical signs range considerably in severity from no signs or mild serous ocular discharge to pneumonia and death. The most severe signs tend to occur in very young or elderly debilitated cats. Concurrent immunosuppressive illness or infection with other respiratory pathogens and opportunistic bacteria also profoundly influences disease severity. Clinical signs include conjunctivitis, serous or mucopurulent ocular and nasal discharge and sneezing, and, less commonly, cough or tachypnea (Figure 23-2, A). Mucopurulent discharges result from secondary bacterial infections with opportunistic pathogens such as Streptococcus spp., Staphylococcus spp., Pasteurella multocida, and Escherichia coli. Ocular and nasal discharges may become crusted, and kittens’ eyes may not open as a result of the sticky exudates; when severe this can be followed by extensive corneal damage and rupture of the globe. Lethargy, inappetence, hypersalivation, and fever may be present in acute infections. FCV and FHV-1 infections may also lead to pharyngitis and laryngitis, which can be accompanied by clinical signs of gagging or obstructive respiratory patterns. Ulcerative glossitis and ulceration of the nasal planum, conjunctiva, and skin is more common and severe with FCV infection but can also be associated with FHV-1 infection (see Figure 23-2, B). Clinical features unique to each infection are outlined next. FIGURE 23-2 Chemosis, mucopurulent ocular and nasal discharge (A) and lingual ulceration (B) in a 6-month-old intact male domestic medium-hair cat with chronic nasal discharge and conjunctivitis. A conjunctival swab specimen tested positive with a PCR assay for FCV RNA and negative for FHV-1, Chlamydia felis, and Mycoplasma spp. DNA. (Courtesy of the University of California, Davis Veterinary Ophthalmology Service.) In neonatal infections caused by FHV-1, damage to upper respiratory epithelium may lead to osteolysis of the nasal turbinates and persistent or recurrent sinusitis and rhinitis. Rarely, neurologic signs and reproductive complications such as abortion and fetal resorption have been observed in infected cats, although it is unclear what role FHV-1 itself plays in the pathogenesis of these clinical manifestations. FHV-1 is an important cause of corneal disease in cats, and has been implicated as a cause of acute and chronic ulcerative (epithelial) keratitis, stromal keratitis, eosinophilic keratitis, corneal sequestra, and uveitis. Stromal keratitis is thought to be an immunopathologic response to persistent viral antigens. The presence of dendritic corneal ulcers is thought to be pathognomonic for FHV-1 infection. However, the role that FHV-1 plays as a cause of eosinophilic keratitis, corneal sequestra, and uveitis requires further study, because a clear association between these abnormalities and the detection of FHV-1 within corneal tissues has not always been present.48 Consequences of ocular disease due to FHV-1 include symblepharon (adhesion of an ulcerated conjunctiva to itself or the cornea) and keratoconjunctivitis sicca. FHV-1 can also cause a severe ulcerative and eosinophilic facial dermatitis (Figure 23-3). Lesions have also been described elsewhere on the body in the absence of facial lesions.49 FIGURE 23-3 Ulcerative and eosinophilic facial dermatitis secondary to FHV-1 infection.A, 3-year-old female spayed domestic shorthair with a 2-month history of a crusted lesion on the right dorsal muzzle. B, Fourteen-year-old female spayed domestic shorthair with blepharokeratoconjunctivitis and severe ulcerative facial dermatitis. C, Same cat as in B after 4 months of treatment with famciclovir. Fluorescein stain is present. Biopsy in both cats showed severe, diffuse, necrotizing, and eosinophilic dermatitis with a few intranuclear inclusions bodies. A PCR assay for FHV-1 DNA on a biopsy from the cat in B was positive. (Courtesy of the University of California, Davis Veterinary Dermatology and Ophthalmology Services.) FCV infection has been most strongly associated with erosive or ulcerative lesions, which can occur on the nasal planum, tongue, lips, and occasionally the conjunctiva and heal over a period of 2 to 3 weeks.50 Persistent infection with FCV has also been linked to chronic ulceroproliferative and lymphoplasmacytic stomatitis, which involves the mucosa lateral to the palatoglossal arches (caudal stomatitis), the alveolar mucosa in the premolar and molar area, and sometimes the buccal mucosa (alveolar/buccal mucositis) (Figure 23-4).51–53 Some persistently infected cats have isolated hyperemia of the buccal mucosa along the length of the dental arcade in the absence of significant periodontal disease. There is no age predisposition for this condition.53 Pyrexia and transient lameness due to synovitis has been described days to weeks after clinical signs of acute FCV infection and after vaccination with certain FCV vaccines. FCV has been investigated as a possible cause of feline lower urinary tract disease (feline interstitial cystitis) and enteritis in cats. Because FCV can be shed in the urine and feces of apparently healthy cats, the significance of the virus in the pathogenesis of these conditions remains unclear. FIGURE 23-4 Severe caudal stomatitis in a retrovirus-negative, 8-year-old female spayed domestic shorthair cat that was evaluated for ptyalism.The cat lived with 12 other cats. Histopathology revealed severe plasmacytic and neutrophilic inflammation with multifocal epithelial ulceration and hyperplasia. Cats with VSD show severe signs of caliciviral URTD, including anorexia, fever (often >105°F [40.6°C]), weight loss, oral and footpad ulceration, and nasal and/or ocular discharge. VSD strains infect not only epithelial cells of the upper respiratory tract and oral cavity, but a variety of other cell types, such as endothelial cells, hepatocytes, pneumocytes, and pancreatic acinar cells.54 FCV uses feline junctional adhesion molecule A (JAM-A) as a receptor, a member of the immunoglobulin superfamily. The lesions that develop are thought to result from disruption of intercellular tight junctions and vasculitis. Distinctive clinical signs of VSD include cutaneous edema, alopecia, crusting, and ulceration. Edema occurs most commonly on the head and limbs but may become generalized. Crusting and ulceration are most prominent on the nose, lips, pinnae, periocular regions, and distal limbs. Severe respiratory distress due to pulmonary edema or pleural effusion, or icterus as a result of hepatic necrosis or pancreatitis, develop in some cats and have been associated with a poor prognosis. Involvement of the gastrointestinal tract, liver, and pancreas may also result in vomiting and/or diarrhea. Cats also develop a coagulopathy, which can be manifested by petechial and ecchymotic hemorrhages and, rarely, epistaxis and hematochezia. In peracute infections, cats die as a result of cardiovascular arrest with few preceding signs apart from fever. In addition to fever, anorexia, lethargy, conjunctivitis, nasal and ocular discharges, and tachypnea as a result of viral pneumonia, highly pathogenic H5N1 avian origin influenza virus infections have been associated with neurologic signs such as seizures and ataxia, which result from nonsuppurative meningoencephalitis and vasculitis.36 Diarrhea and vomiting have not been observed in cats infected with H5N1 viruses. Vomiting has been reported in cats infected with pandemic H1N1 influenza viruses, which may have a predilection for the gastrointestinal tract.33,34 Physical examination findings in cats with acute viral URTD vary from mild serous ocular discharge and conjunctivitis through to fever, severe mucopurulent ocular and nasal discharges, chemosis, dehydration, thin body condition, stertorous or stridorous respiration, tachypnea, increased breath sounds on thoracic auscultation, hypersalivation, and ulceration of the nasal planum, tongue, and lips. Because ulcerative lesions can occur at the base of the tongue near the larynx, examination of the entire tongue should be performed in cats that are febrile and inappetent, which may require sedation. Cats with VSD can have edema of the face and lips, icterus, cutaneous ulceration, evidence of petechial hemorrhages, and abdominal pain. Ulcerative keratitis with dendritic or geographical corneal ulceration may be seen in cats with acute FHV-1 infection; chronic FHV-1 infection may be manifested as stromal keratitis with neovascularization, pigmentation and fibrosis of the cornea, symblepharon, and conjunctivalization of the cornea (Figure 23-5). Eosinophilic keratitis manifests as superficial, pink to white vascularized proliferative lesions on the cornea or conjunctiva; abundant eosinophils are present in smears of corneal scrapings. Herpetic facial dermatitis is manifested as cutaneous ulceration, erythema, exudation, and adherent crusts, most commonly around the nose and eyes but occasionally on the trunk and limbs.55 Cats with persistent FCV infection may have ulceroproliferative caudal stomatitis or buccal/alveolar mucositis, and exhibit pain on examination of the oral cavity. FIGURE 23-5 A, Ulcerative and stromal keratitis in a 2-year-old male neutered rex cat. FHV-1 infection was suspected. Treatment with famciclovir (375 mg PO q8h), l-lysine (500 mg PO q12h), and cidofovir (0.5%; one drop OD q12h) was associated with clinical improvement over a 1 month period (B and C). The cat had a history of a corneal sequestrum and subsequently developed eosinophilic keratitis. (Courtesy of the University of California, Davis Veterinary Ophthalmology Service.) There are no specific CBC, biochemistry profile, or urinalysis abnormalities that aid in a diagnosis of feline viral respiratory disease. The CBC can be normal or show a mild to moderate neutrophilia, sometimes with band neutrophils or neutrophil toxicity. Lymphopenia may be present in severely affected cats. Serum biochemistry findings in cats with viral respiratory disease are usually unremarkable unless disease is severe or chronic. In cats with VSD, hematologic abnormalities include mild to severe anemia, thrombocytopenia, neutrophilia, and lymphopenia. Cats with VSD may also have hypoalbuminemia, hyperbilirubinemia, mildly increased serum activities of ALT and AST, and increased serum CK activity. Serum CK activities up to 11,000 U/L can occur.26 Cats with chronic stomatitis may have hyperglobulinemia due to a polyclonal gammopathy. Tracheobronchial lavage specimens from cats with pneumonia may show a suppurative or mixed exudate, sometimes with evidence of secondary bacterial infection. Aerobic bacterial culture and susceptibility testing and culture for Mycoplasma spp. are indicated on wash specimens. Organisms such as Pasteurella spp., Staphylococcus pseudintermedius, Streptococcus spp., Escherichia coli, Klebsiella pneumoniae, and some Mycoplasma spp. infect the airways as opportunists. Bacterial culture of nasal swabs is generally not recommended because it often leads to growth of normal flora, which has no clinical relevance. However, resistant Pseudomonas aeruginosa infections can develop in some cats with chronic URTD that have been treated repeatedly with antimicrobial drugs.
Feline Respiratory Viral Infections
Etiology and Epidemiology

Feline Herpesvirus-1
Feline Calicivirus
Influenza Viruses
Clinical Features
Feline Herpesvirus-1
Feline Calicivirus
Influenza Virus Infections
Physical Examination Findings
Diagnosis
Laboratory Abnormalities
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree