31 Exercise-associated muscle disorders
Muscular disorders unfortunately are not uncommon in the exercising horse, or one that has completed an exercise bout in the last 24 hours or so. These conditions may be associated with a traumatic incidence or the exercise itself. The development of acute muscle pain following exercise, for example, can occur in horses performing strenuous exercise beyond their training adaptation, or as a consequence of repetitive motion injuries (including overtraining/over-use). A training imbalance therefore should be suspected if there is a history of an increase in work intensity without appropriate training, too quick/early return to work following a period of rest, over-training or overload, etc. Such muscular disorders are not commonly associated with a nutritional imbalance and although an appropriate training plus dietary regimen are important in their management they will not be discussed in any further detail in this chapter. Similarly although muscular stiffness and a reluctance to move can be associated with the exhausted horse syndrome this condition will not be discussed in any detail neither will muscle pain or myalgia potentially associated with influenza and other viral diseases. Even though it is not associated with structured exercise, as major outbreaks of atypical myoglobinuria have been reported in Europe and midwestern USA in the recent past a few key points about this condition are given in Box 31.1. This chapter, therefore, concentrates on the equine rhabdomyolysis syndrome (ERS).
Box 31.1
Atypical Myoglobinuria or Atypical Myopathy
(Whitwell et al 1998, Van der Kolk et al 2010, Van Galen et al 2012 a,b, Votion 2011).
• Typically seen in horses and ponies out at grass, on a low plain of nutrition. Usually not being worked or in light work.
• Sudden onset – often found dead or recumbent – less severe may present with stiffness unrelated to exercise that may rapidly progress to recumbency.
• Metabolic defect may be a multiple acyl-coenzyme A dehydrogenase deficiency, which affects mitochondrial fatty acid energy metabolism. But development is probably multifactorial and may involve mycotoxins/clostridial toxins in some instances.
• Often preceded by adverse (cold, wet, windy, etc. but no heavy frost) weather conditions. Large outbreaks seem to have occurred following summers, and during autumns, that were significantly warmer than usual.
• One or more animals affected within a group. Others appear to be totally unaffected (but some may be subclinical). Mortality high and can be up to 100% on one pasture. Overall recently estimated at 76%.
• Variable ages – but young (<3 years) and old (>20 years) appear more at risk.
• Usually do not appear to be in distress or pain and will often eat and drink normally (some even appear to be starving) if have access.
• Pulse, temperature, respiration rates can be within acceptable normal limits, even when recumbent, but tachycardia and tachypnea have been reported.
• Can develop respiratory, cardiac, renal and digestive complications.
• CK/AST activities markedly elevated: 10–100 s ×103 IU/l.
• Mygolobinuria, ± hypocalcemia (and low ionized calcium especially in terminal stages).
• Inconsistent changes in liver enzymes, Se or glutathione peroxidase (GSHpx) or liver vitamin E levels.
• Biopsy samples taken from postural and/or respiratory muscles and/or myocardium typically show acute segmental necrosis of striated muscle fibers without mineralization.
• No specific treatment currently available (although if associated with Clostridum toxin appropriate treatment may be helpful).
• Supportive treatment includes pain management, fluid therapy, administration of calcium, vitamins and antioxidants, plus enhancement of carbohydrate metabolism.
• Recommended to also provide support to apparently unaffected pasture companions (rest, reduce stress, nutritional support, etc.).
• Recent epidemiological work has suggested certain factors within the individual and the environment that seem to increase (e.g., being out at pasture all year, pasture on a steep slope, surrounded by or containing a stream/river or trees especially Acer pseudoplatanus, being given hay in fall) or decrease the risk (e.g., being overweight, and regularly vaccinated and given anthelmintic treatment, water provided by a distribution network).
• Certain preventative measures may be helpful especially for pastures with a prior history of deaths (e.g., reduce time out on pasture in spring and autumn, or avoid totally if surrounded by trees especially Acer pseudoplatanus, provide water from a distribution network, vaccinate horses regularly, do not allow to get underweight, etc.).
The ERS affects primarily the muscles of horses of apparently any age, breed or gender and results in the partial or complete inability to move (e.g., signs may range from a show pony that may fail to lengthen when asked, or a racehorse that slows in the closing stages, to an animal that cannot move, or becomes recumbent: Harris 1991). Death can result, although this is rare. The time period between episodes and the severity of the episodes vary between and within individuals (Harris 1991).
Epidemiology and risk factors
ERS according to a number of surveys can affect approximately 5–7% of the thoroughbred racehorse – flat and National Hunt – population, ~8% of polo horses and up to 14% of eventers (MacLeay et al 1999a, McGowan et al 2002a, b, Cole et al 2004, Upjohn et al 2005, Thorpe & Harris 2005). A survey of pleasure horses in Scotland (Mellor et al 2001) suggested a prevalence of 1% and a general survey in Australia concluded that 1.9% of the general equine population over 1 year of age had suffered one or more episodes of ERS during the previous 12 months (Cole et al 2004). In this survey, exercising animals were estimated to be more than 10 times as likely to suffer an episode compared to those not being exercised.
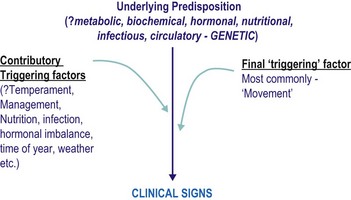
Sufferers have an underlying susceptibility to the condition, which may then be triggered by one or more factors, usually including exercise, resulting in the clinical signs (see Fig. 31.1). The underlying predispositions, as well as the triggering factors, are likely to differ between groups of sufferers – so the measures that may be successful in one individual may not be so successful in another. Sufferers currently may be divided into two groups:
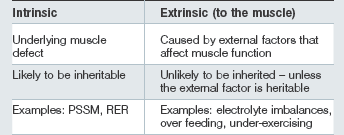
• Those in which the primary underlying susceptibility is intrinsic to the muscle: sometimes referred to as chronic.
• Those in which an intrinsic muscle defect does not appear to be present (or to date we have not been able to show that an intrinsic muscle defect is present): sometimes referred to as sporadic.
Classification and etiology
An intrinsic muscle defect is not present
Cases of nonintrinsic ERS are usually characterized by a history of adequate performance prior to episodes developing and a successful return to performance following a reasonable period of rest, provision of a balanced, appropriate diet and an appropriate training program. Horses may be of any age, breed or sex and involved in a wide variety of athletic disciplines. Horses can have recurrent episodes of variable severity with inconsistent intervals between them if appropriate changes in the diet and management, etc. are not made (Harris 1991). Episodes of ERS are brought about by external factors which affect muscle function and once corrected complete resolution is possible. In the majority of cases, horses should initially be thought to have nonintrinsic ERS; however, possible intrinsic muscle defects need to be considered if over time multiple episodes of ERS occur despite appropriate management (training and feeding).
• Exercising after a period of over feeding and under exercising.
• Electrolyte imbalances (dietary or individual animal in origin) (see below).
• Provision of too little fiber and too much nonstructural carbohydrate (NSC).
An initial dietary evaluation determining the approximate daily NSC, oil and forage intake as well as the vitamin, mineral and electrolyte balance may suggest dietary imbalance as a contributing cause. If imbalances appear to exist, a full ration evaluation may be necessary including analysis of hay (or pasture grass) and all complementary and supplementary feeds. Some individuals despite an apparently balanced and adequate intake of electrolytes may have an electrolyte imbalance when assessed by use of fractional urinary electrolyte clearance measurements (Harris & Colles 1988, Harris & Snow 1991, Harris & Gray 1992).
Vitamin E and selenium deficiency
Evidence for a role of vitamin E/Se deficiency in the pathophysiology of ERS has been based on anecdotal reports of supplementation preventing further episodes (Hill 1962, Mansmann et al 1982). These anecdotal findings have not been supported to date by scientific studies. Vitamin E/Se responsive myopathies of sheep and pigs have selective Type I fiber degeneration as compared to the Type II fiber involvement in ERS. However, little is known about the role of free radical induced muscular damage in ERS, delayed onset muscle soreness or even over exertion (Harris & Mayhew 1998).
Electrolyte imbalances
Within this chapter an electrolyte imbalance, as it relates to an individual horse, is taken to represent an inadequate or imbalanced intake or an individual problem with absorption/utilization. It is possible that changes in the intracellular environment within muscle associated with such an electrolyte imbalance may be important. However, there is little published work in this area (Bain & Merritt 1990, Harris & Snow 1991).
Whilst an electrolyte imbalance within the diet may be identified through dietary analysis, an issue within an individual animal may be difficult to determine accurately, One practical way may be to measure urinary fractional excretion (FE) of electrolytes (Harris & Colles 1988, Harris & Gray 1992, McKenzie et al 2002, 2003a), although this at best is only a guide because marked variation can occur due to differences in the core diet, effect of exercise, sampling technique and between individuals as well as within individuals from day to day. Averaging the results of freely voided urine taken on three consecutive days under the same conditions, at the same time of day (pre-exercise and before or >8 h after a concentrate meal, at least 2 weeks after an episode, having been fed a standard ration thought to provide adequate and balanced intake of electrolytes for at least 2 weeks) may increase accuracy. Furthermore, the high calcium crystal concentration of alkaline equine urine requires acidification to accurately assess Ca and Mg content, the high potassium content can interfere with sodium analysis using conventional ion-specific electrodes and results are likely to be unreliable if the urine creatinine concentration is <9000 µmol/l, the pH is 6 or less, blood urea/creatinine are elevated, or there is glucose/hemoglobin present in the urine (Harris & Gray 1992).
Animals without renal disease, that have abnormal FE values while being fed a diet that should provide an adequate and balanced electrolyte content, may have an individual absorption/utilization problem. Such abnormalities (e.g., low or high FE Na values, raised FE PO4) have been found in some horses and ponies suffering from musculoskeletal problems, in particular the equine rhabdomyolysis syndrome (Harris & Colles 1988, Harris & Snow 1991). Restoration of the FE values to within the expected reference range for the type of diet fed may result in clinical improvement in some but not all animals (see Harris & Snow 1991). It should be noted that no differences in electrolyte status as determined by the FE test was found between thoroughbred horses confirmed to have the intrinsic muscle defect (RER) and control horses (McKenzie et al 2002).
Intrinsic muscle defect is present
Some horses develop ERS repeatedly with very little exertion and despite reasonable management practices. Signs are often first apparent at the beginning of training or when horses have developed a reasonable level of fitness or are being fed over a certain level of cereal-based concentrates. Certain breeds of horses appear to have a higher prevalence of ERS and within these breeds specific family lines appear particularly predisposed. This has led to the suggestion that, similar to humans, there are intrinsic inherited defects in muscle function which may predispose horses to such forms of ERS. Documented forms of “intrinsic-defect” ERS include a disorder in muscle contractility or excitation–contraction coupling, which is often referred to as recurrent exertional rhabdomyolysis (RER; Box 31.2) and a defect in carbohydrate storage and/or utilization (polysaccharide storage myopathy [PSSM; Box 31.3]). Other types most probably exist. In both current subgroups a lack of routine daily exercise and a diet high in starch are common predisposing factors for an episode.
Box 31.2
Recurrent Exertional Rhabdomyolysis
(Beech et al 1993, Lopez et al 1995, Lentz et al 1999a,b, 2002, MacLeay et al 1999a, b, c, 2000, Ward et al 2000, Mlekoday et al 2001, Dranchak et al 2005).
• Currently thought to be due to an abnormality in the process of muscle contraction (e.g., the mechanism by which muscle contraction is regulated can be disrupted by excitement and exercise in some susceptible horses). Most likely due to a defect in the regulation of muscle contractility (increased sensitivity of muscle fiber bundles has been shown in vitro to potassium, caffeine and halothane) potentially caused by a defect in skeletal muscle calcium regulation. Recent work, however, suggests that this condition is likely to be caused by a gene/gene alteration that is not yet known to cause similar muscle disease in other species.
• Found mainly in Thoroughbreds, Standardbreds and Arabians.
• RER is reported most frequently in young (two-year-old) fillies in race training. The sex predilection for females does not appear to be as obvious in older horses with RER.
• Commonly suggested triggering factors include:
• Incidence of RER may become more frequent as level of fitness increases. Stress is a very common triggering factor. In Standardbreds, ER seems to occur most often after 10–30 min of jogging.
• Horses with RER are typically described as having a nervous temperament and clinical episodes of ER often occur when exercise is accompanied by excitement.
• Most likely to be an inherited condition (possibly autosomal dominant trait with variable expression). It has been suggested that foals of an RER affected stallion or mare would have have at least a 50% chance of inheriting the RER gene. NB this is modifiable by environmental factors.
Box 31.3
PSSM
(Valberg et al 1992, 1998, 1999a,b, Valentine et al 1997, 2000, 2001, 2006, Valentine & Cooper 2005, Quiroz-Rothe et al 2002, Annadale et al 2004, 2005, McCue et al. 2006, 2008a,b, 2009a,b, Hunt et al. 2008, Larcher et al. 2008, Barrey et al 2009, Stanley & Piercy 2007, Herszberg et al 2009, Stanley et al 2009).
• The clinical condition is mainly found in Quarter horses and related breeds (e.g., Paints, Appaloosa), Warmbloods and Morgans. Also reported in Anglo-Arabs and Andalusians as well as cob types in the UK. PSSM does not commonly occur in the thoroughbred racehorse if it occurs at all.
• Recent survey suggested that the prevalence of PSSM among overtly healthy Quarter Horses in the US was likely to be between 6 and 12%.
• A prevalence of 33% of all horses outside of draft and Quarter Horse bloodlines and up to 80% of draft horses has been suggested for polysaccharide storage myopathy when amylase-resistant abnormal-polysaccharide is not a required diagnostic criterion.
• Feeding a meal with a high glycemic index produces a lower glucose and insulin response in PSSM Quarter horses compared to normal horses. Thus it appears that such PSSM horses may store a higher proportion of the absorbed glucose after a starch meal in their muscle compared to normal horses. No limitations in the ability of skeletal muscle to metabolize glycogen have been identified in PSSM horses performing anaerobic exercise. However, during submaximal exercise, a defect in substrate flux during metabolism is suggested by stimulation of purine nucleotide metabolism in muscle of PSSM but not healthy horses after 10 min of walk and trot. Muscle cramping with light exercise in PSSM horses may be a result of this defective energy generation in individual muscle fibers.
• In Quarter horses it appears to be associated with abnormal insulin sensitivity but this has not been found in Belgian Warmbloods with PSSM, suggesting that PSSM in these animals may be associated with excessive glycogen synthesis rather than enhanced glucose uptake into muscle cells.
• A missense gain of function mutation (arginine to histidine substitution) in the equine glycogen synthase 1 gene (GYS1) has relatively recently been reported in PSSM-affected Quarter Horses, draughts and a variety of other breeds. This mutation causes abnormal increased glycogen synthase activity in skeletal muscle at rest and when activated by glucose-6-phosphate. Hence the increased ratio (of glycogen synthase to branching enzyme activity) in GYS1-mutated muscle likely causes abnormal filamentous polysaccharide inclusions to form. This is now referred to as type 1 PSSM to distinguish from those forms of PSSM that are not associated with this genetic mutation (type2 PSSM).
• The precise defect causing the different forms of PSSM is unknown despite numerous biochemical studies. Recent work, at least in the Norman Cob horse breed, concluded that the main disorders seen “could be related to mitochondrial dysfunctions, glycogenesis inhibition and the chronic hypoxia of the PSSM muscles.” It has also been suggested that the persistent glycogen synthase activity in type 1 PSSM muscle adversely affects normal muscle energy metabolism during exercise but the actual link between diet, enhanced glycogen synthase activity and muscle cell damage has not been fully elucidated.
• Trigger factors include a change in the exercise routine including unaccustomed stall confinement, being rested for a few days prior to exercise, infection and most importantly the diet. When signs first occur tends to be influenced by the amount of exercise and dietary starch fed.
• Sufferers of this subgroup of ERS tend to have a more calm temperament than the other subgroup and often have persistent elevations of creatine kinase (CK) without these always being associated with clinical signs especially if stable confined. Horses typically are in good (and sometimes overweight) body condition.
• In the Quarter horses and related breeds, and in one warmblood family at least, there appears to be a hereditary basis for PSSM with a dominant mode of inheritance of the GYS1 mutation although clinical signs of ERS are not always present (depending at least in part on environmental factors).
Clinical signs
Clinical signs usually occur either during or after some form of exercise (as the animal returns home or in the stable/field after exercise). A small number of cases, however, have been reported when the animal is not in work or as it leaves the stable/field at the start of exercise. The main clinical sign is some degree of muscular stiffness, which can be very mild or result in a total inability to move. Recumbency can occur and on rare occasions the condition is fatal. The hind limbs are most commonly affected, usually bilaterally. Firm, swollen muscle groups may be present, especially in the more severely affected animals, but this is not always the case. Palpation of the muscles may or may not be resented. Signs associated with pain and distress may be apparent to a variable extent and attempts have been made to grade the severity of clinical signs (Harris 1991
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree