CHAPTER 14 Equine Viral Arteritis
Equine viral arteritis (EVA) is an infectious disease of equids that is caused by equine arteritis virus (EAV). EAV infection occurs throughout much of the world, although the incidence of both EAV infection and clinical EVA varies greatly between countries and among horses of different breeds. The vast majority of EAV infections are inapparent or subclinical, but occasional outbreaks of EVA are characterized by any combination of influenza-like illness in adult horses, abortion in pregnant mares, and interstitial pneumonia in very young foals.1,2
An extensive outbreak of EVA that occurred in Kentucky Thoroughbreds in 1984 generated widespread interest, publicity, and concern.3–6 Since then, a number of other outbreaks have been reported from North America and Europe.6–11 Similarly, EAV infection of horses has recently been identified in countries such as Australia, New Zealand, and South Africa that were previously thought to be largely or completely free of the virus.12–17 This apparent global dissemination of EAV and rising incidence of EVA likely reflect the rapid national and international movement of horses for competition and breeding, as well as increased recognition of the importance of EAV infection.3,18–22
ETIOLOGY
EAV was first isolated from the lung of an aborted fetus after an extensive outbreak of respiratory disease and abortion on a Standardbred breeding farm near Bucyrus, Ohio, in 1953.23,24 EVA was identified as an etiologically distinct disease after isolation of the causative virus (EAV) and description of characteristic vascular lesions.25 EVA was distinguished from equine influenza and equine herpesviruses type 1 and type 4 (EHV-1, EHV-4), which potentially cause similar respiratory and reproductive disease syndromes in horses.23,24 Although it was not definitively confirmed to be a new disease entity until 1953, apparent EVA was described in the late eighteenth and early nineteenth centuries as “pinkeye,” “infectious or epizootic cellulitis,” “influenza erysipelatosa,” “Pferdestaupe,” “Rotlaufseuche,” and “equine influenza.”26–29
Genome Organization and Virion Structure
EAV is the prototype virus in the family Arteriviridae (genus Arterivirus, order Nidovirales), a grouping that also includes porcine reproductive and respiratory syndrome virus, simian hemorrhagic fever virus, and lactate dehydrogenase–elevating virus of mice.30,31 The EAV virion is an enveloped, spherical, 50- to 65-nm particle with an icosahedral core that contains a single-stranded, positive-sense ribonucleic acid (RNA) molecule of about 12.7 kilobases31–33 (Fig. 14-1). The EAV genome includes a 5′ leader sequence and nine open reading frames (ORFs).32,34 The two most 5′-proximal ORFs (1a and 1b) occupy approximately three fourths of the genome and encode two replicase polyproteins (pp1a and pp1ab). These two precursor proteins are extensively processed after translation into at least 12 nonstructural proteins (nsp 1-12) by three viral proteases (nsp1, nsp2, and nsp4).31,35,36 The greatest variation in the EAV replicase gene occurs in the portion of ORF1a encoding the nsp2 protein, with considerable variation at amino acids 388 to 488.37
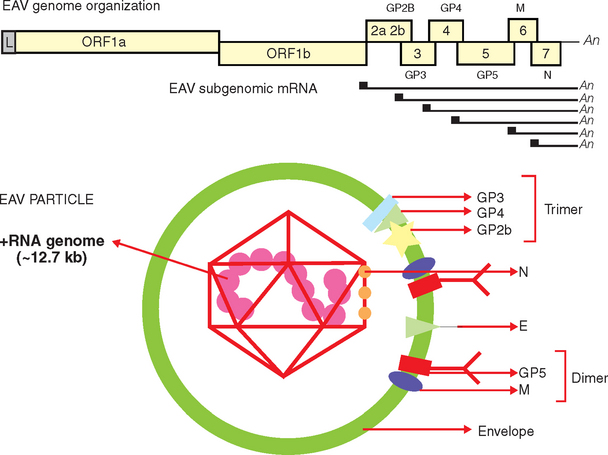
Fig. 14-1 Schematic representation of equine arteritis virus (EAV) genome organization and the virus particle.
The structural proteins of the EAV virion include six envelope proteins (E, GP2b [GS], GP3, GP4, GP5 [GL], and M) and a nucleocapsid protein (N), which, respectively, are encoded by ORFs 2a, 2b, and 3 to 7 located at the 3′ proximal quarter of the genome.33,38–40 These structural proteins are expressed from six subgenomic viral messenger RNAs (mRNAs) that form a 3′-coterminal nested set and contain a common leader sequence of 224 nucleotides encoded by the 5′ end of the genome. Three of the minor envelope proteins (GP2b, GP3, and GP4) form a heterotrimer in the EAV particle, and the M and GP5 proteins form a disulfide-linked heterodimer.40–42
The greatest sequence variation in the ORFs encoding structural EAV proteins occurs in ORFs 3 and 5, which encode GP3 and GP5, respectively.37,43–46 GP5 expresses the major neutralization determinants of EAV, and although considerable variation exists in the sequence of the GP5 protein of field strains of the virus, there is only one serotype of EAV, and all strains evaluated thus far are neutralized by polyclonal antiserum raised against the virulent Bucyrus strain.45,47–53 However, field strains of EAV are frequently distinguished based on their neutralization phenotype with polyclonal antisera and monoclonal antibodies. Similarly, geographically and temporally distinct strains of EAV differ in the severity of the clinical disease they induce and in their abortigenic potential.1,2,54–58 Although strains of EAV from North America and Europe share as much as 85% nucleotide identity,37,45,57 these viruses generally segregate into clusters reflective of their geographic origins after phylogenetic analysis.45,46
EPIDEMIOLOGY
Seroprevalence and Breed Predilection
EVA is a disease of the horse, but antibodies to EAV recently have been identified in donkeys in South Africa.15,59 Serologic surveys have shown that EAV infection occurs among horses in North and South America, Europe, Australia, Africa, and Asia.1 However, the seroprevalence of EAV infection of horses varies between countries and among equine populations within some countries. Iceland and Japan, for example, are apparently free of the virus. EAV infection is relatively common in horses in a number of European countries; studies conducted in 1973 estimated the seroprevalence of EAV infection at 11.3% of Swiss horses and 2.3% of English horses.60,61 Similarly, approximately 14% of Dutch horses were seropositive to EAV in surveys done in 1963 and 1975.60 In German horses, 1.8% were seropositive in a study conducted in 1987, and the seroprevalence increased to 20% in a subsequent survey in 1994.62 In the United States (U.S.), the 1998 National Animal Health Monitoring System (NAHMS) equine survey showed that only 2.0% of unvaccinated horses in the U.S. were seropositive to EAV.63 Similarly, resident unvaccinated California horses had a seroprevalence to EAV of only 1.9%, whereas 18.6% of horses imported into California from other countries (most often European Warmbloods) were seropositive.64
The seroprevalence of EAV infection varies not only between countries but also among horses of different breed and age, with marked disparity between the prevalence of infection of Standardbred and Thoroughbred horses.65,66 EAV infection is considered endemic in Standardbred but not Thoroughbred horses in the U.S., with 77.5% to 84.3% of all Standardbreds but only up to 5.4% of Thoroughbreds being seropositive to the virus.1,66–70 Similarly, the seroprevalence of EAV infection of Standardbred horses in California was 68.5% in 1991, versus less than 2% in all other breeds tested.68 The seroprevalence of EAV infection of Warmblood stallions is also very high in a number of European countries; about 55 to 93% of Austrian Warmblood stallions are positive for antibodies to EAV.71
These profound differences in the breed-specific seroprevalence of EAV infection might reflect inherent genetic differences that confer resistance to infection. More likely, however, these differences reflect the different management practices used with individual horse breeds. Specifically, studies have not demonstrated any breed-specific variation in susceptibility to EAV infection or in establishment of the carrier state;72 thus the number of actively shedding carrier stallions likely determines the prevalence of EAV infection in individual horse breeds. The seroprevalence of EAV infection increases with age, indicating that horses may be repeatedly exposed with increasing age.71,73
Outbreaks
Since the recognition of EVA in 1953, outbreaks of the disease have been reported from Switzerland,74,75 Austria,76,77 Poland,78,79 Italy,80,81 the United Kingdom,6,8–10 Spain,7 Netherlands,82 Canada,83,84 and the United States.1,2,56,67,85–88 At least four major documented outbreaks of EVA have occurred in the U.S. since the 1953 epizootic,54,86–88 the first at a racetrack in Kentucky in 1977.87 Subsequent epizootics included an extensive outbreak in Thoroughbred horses in central Kentucky during the l984 breeding season88 and another in racing Thoroughbred horses in 1993 (>200 clinical cases) that began at the Arlington racetrack in Chicago and then spread to horses at Churchill Downs, Prairie Meadow, and Ak-Sar-Ben.86 A well-documented outbreak of EVA occurred on a single Warmblood breeding farm in Pennsylvania in 1996, precipitated by an imported carrier stallion.2,54 Similarly, the first recorded outbreak of EVA in the United Kingdom followed the importation of an Anglo-Arab stallion from Poland.8,70,89
Transmission
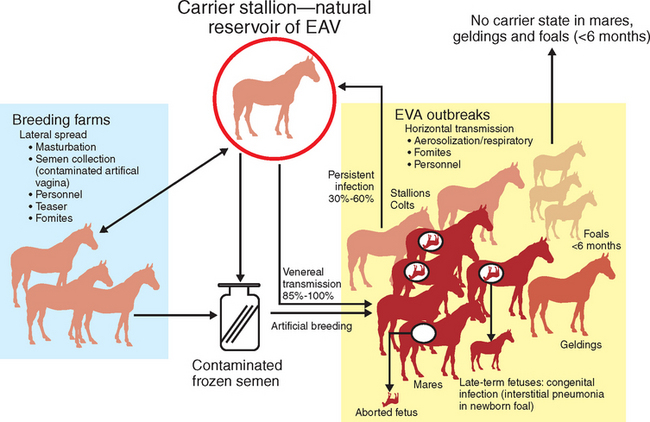
Transmission of EAV between horses occurs through either respiratory or venereal routes1,24,65,90–92 (Fig. 14-2). Horizontal respiratory transmission of EAV occurs after aerosolization of infected respiratory tract secretions from acutely infected horses; high titers of EAV are present in respiratory secretions for some 7 to 14 days during acute infection.91 However, direct and close contact is necessary for aerosol transmission of EAV between horses.66,85 EAV also can be transmitted by aerosol from urine and other body secretions of acutely infected horses, aborted fetuses and their membranes, and the masturbates of acutely or chronically infected stallions.16,61,71,91,93–95 Venereal transmission of EAV contained in the semen of stallions that are either acutely or chronically infected with EAV is the other important route of natural transmission of the virus.65,92
Persistently infected carrier stallions are the essential reservoir responsible for perpetuation and maintenance of EAV in equine populations. The persistent EAV infection that occurs in carrier stallions is highly unusual in that virus is shed only in the reproductive tract; thus, 85% to 100% of seronegative mares bred to long-term carrier stallions become infected with the virus and seroconvert within 28 days. Mares are also readily infected by artificial insemination with semen collected from shedding stallions.56 Mares that become infected following natural or artificial breeding then can readily transmit the virus by nasal aerosol to susceptible cohorts in close proximity.90
Other, less common modes of transmission of EAV include congenital infection of foals after transplacental transmission of the virus in mares infected in late gestation.96 The virus is not teratogenic, but congenitally infected foals may develop a rapidly progressive, fulminating interstitial pneumonia and fibronecrotic enteritis.79,96–99 Lateral dissemination of EAV also can occur through fomites (e.g., personnel, clothing, vehicles, equipment),1,61,66,85 as described during an outbreak of EVA on a Warmblood breeding farm in Pennsylvania, where virus was first indirectly spread from a carrier stallion to a susceptible horse.54 The virus then rapidly spread by aerosol to contact horses. Similarly, EAV was spread among nonbreeding Lipizzaner stallions in South Africa by the apparent aerosolization of virus shed into bedding in the masturbates of a carrier stallion(s).26
Carrier State and Molecular Epidemiology
The asymptomatic carrier stallion is the essential natural reservoir of EAV, as first described more than 100 years ago when it was noted that healthy stallions could transmit so-called epizootic cellulitis-pinkeye or influenza (which likely was EVA) to mares at breeding.28,29 The EAV carrier state was poorly defined until the 1984 epizootic in Kentucky, when studies by Timoney and McCollum unequivocally established the importance of the carrier stallion in the natural epidemiology of EAV infection.65,92,100,101 Specifically, Timoney et al.92 confirmed the chronic carrier state in naturally infected Thoroughbred stallions using test matings and isolation of virus from semen and showed that 30% to 35% of stallions that were infected during the outbreak subsequently became long-term carriers.
Persistently infected stallions can be divided into three groups based on the duration of virus excretion in semen.65,102 The short-term, or convalescent, carrier state lasts only a few weeks after clinical recovery, and the intermediate carrier state lasts for 3 to 7 months in both naturally and experimentally infected animals. The long-term, or chronic, carrier state can last for years and even the entire life of the infected stallion. Some persistently infected, long-term carrier stallions cease to shed virus after years of persistent infection, with no apparent later reversion to a shedding state. However, the mechanism responsible for this spontaneous clearance of EAV from persistently infected stallions is not clear. There is no convincing evidence that carrier stallions are or can become intermittent shedders of the virus or have latent infection.
Carrier stallions have moderate to high titers of serum neutralizing antibody to EAV and shed the virus constantly in their semen, but virus is not present in their blood, urine, or body secretions.1 EAV appears to be restricted to the reproductive tract during persistent infection of carrier stallions, and highest titers of the virus consistently have been demonstrated in the ampulla of the vas deferens (>105 PFU/g tissue).1,101 Virus in semen is associated with the sperm-rich fraction and not with the pre-ejaculatory fluid, and the titers of virus in sequential ejaculates vary little from the same stallion.
The mechanism of persistence of EAV in the male reproductive tract is not clear. However, studies have established that persistence of EAV in stallions is testosterone– dependent;103,104 persistently infected stallions that were castrated and treated with testosterone continued to shed the virus in semen, whereas untreated animals ceased shedding virus. Holyoak et al.100 studied the persistence of EAV in prepubertal and peripubertal colts and showed that EAV replicates in the male reproductive tract of a significant proportion of colts for a variable period (up to 6 months) after clinical recovery in the absence of circulating concentrations of testosterone equivalent to those found in sexually mature stallions. However, long-term persistent EAV infection does not occur in colts exposed to the virus before the onset of puberty. Similarly, persistent infection does not occur after EAV infection of mares, geldings, or fetuses.1,104 Thus, EAV was not isolated from the reproductive tract of seropositive mares 1 month after infection,104,105 and convalescent mares did not transmit infection to susceptible stallions during mating or to contact horses.66,71,106
The carrier stallion clearly is responsible for generating the genetic heterogeneity that distinguishes individual field strains of EAV. Sequence analyses of the variable ORF5 gene of strains of EAV sequentially present in the semen of carrier stallions showed that EAV behaves as a quasispecies (population of genetically related viral variants) during persistent infection, leading to both genetic and phenotypic divergence of the virus.37,44,54 Outbreaks of EVA result from the emergence and spread of specific variants of EAV that are present in the quasispecies virus population in the semen of individual carrier stallions; however, the mechanisms involved in selection and emergence of virulent viral variants remain unclear. It has also been recently shown that novel variants with distinct neutralization phenotype arise during persistent infection of carrier stallions, and that the altered neutralization phenotype of these variants correlates with amino acid changes in specific regions of the GP5 envelope glycoprotein.44, 47–49,54,56 However, all the variants that arise in the course of persistent infection of carrier stallions are neutralized by high-titer polyclonal equine sera, which suggests that immune evasion is not responsible for the establishment of persistent EAV infection of carrier stallions. There also is no evidence that positive selective pressures are responsible for establishment of persistent EAV infection of stallions.37,44
The recent advent of molecular techniques has greatly increased our understanding of the epidemiology of EVA. For example, investigation of an extensive outbreak of EVA on a Warmblood breeding farm showed that a single virus variant present in the semen of a carrier stallion was selected and then efficiently transmitted by aerosol among other horses on the farm.54 Thus, it appears that the considerable genetic heterogeneity afforded by the viral quasispecies likely facilitates persistence of EAV in the reproductive tract of carrier stallions. However, only some members (variants) within the quasispecies appear to be capable of efficient aerosol transmission to other horses, perhaps because of an enhanced ability to replicate within the respiratory tract. The strain of EAV that circulated during this particular outbreak was genetically stable during repeated horizontal and vertical passage in horses, unlike the diverse, quasispecies virus population in the semen of the carrier stallions on the farm.
PATHOGENESIS
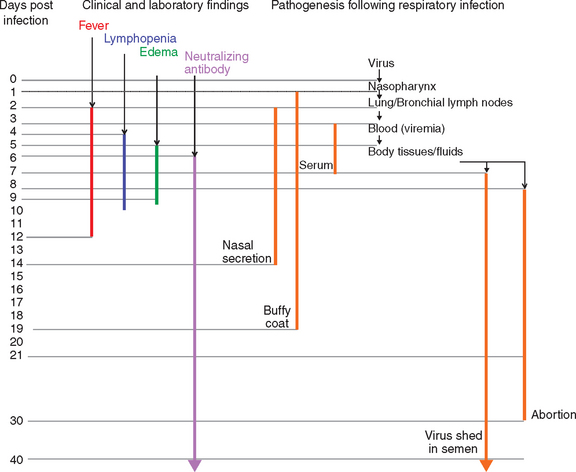
The pathogenesis of EVA has been studied by both the experimental inoculation (intranasal, intramuscular, intravenous) of horses with strains of EAV of different virulence and the careful evaluation of natural outbreaks of EVA.2,58,90,91,107–113 Quantitative distribution of EAV has differed greatly between individual experiments, which likely reflects the inherent differences in the route of infection, virus dose, strain of virus, and quality of the specimens used for virus isolation.33 Briefly, EAV is rapidly spread within the lung and bronchial lymph nodes (in 2 days) after aerosol infection, then is disseminated throughout the body through the circulation. Virus can be isolated from the nasopharynx, buffy coat, and serum for a variable time after intranasal exposure (Fig. 14-3). Virus can be isolated from the nasopharynx for 2 to 14 days after infection and from buffy coat for 2 to 19 days. Virus typically is isolated from serum or plasma for 7 to 9 days, and the disappearance of virus from serum coincides with the development of virus-specific neutralizing antibodies. Virus can be isolated from a wide variety of tissues and body fluids of infected horses beginning about 1 to 2 days after infection.91 Apart from occasional cases where EAV has been isolated from buffy coat cells for several months after infection, and from the reproductive tract of prepubertal colts (≤6 months of age), EAV generally is not isolated beyond 28 days after infection except from the semen of carrier stallions.
The pathogenesis of EVA is not clearly defined. Many of the clinical manifestations of EVA result from vascular injury, and death in horses inoculated with the highly virulent, horse-adapted Bucyrus strain of EAV is a consequence of severe vascular damage leading to disseminated intravascular coagulation. The characteristic vascular lesions of EVA have been compared to those of Aleutian disease of mink and other immune-mediated vascular diseases.114,115 The lesions of EVA, however, do not appear to be the result of immune-mediated injury, because they develop at only 4 to 5 days after experimental inoculation, which is not consistent with an immune-mediated process. Furthermore, arteries larger than 1 mm are affected, and neither immunoglobulin G (IgG) nor complement (C3) is present in the lesions, as would be expected if immune complexes were responsible.
Therefore, vascular injury in EVA likely results from direct virus-mediated injury to the lining (endothelium) and walls (media) of affected vessels. EAV infects and replicates in endothelial cells (ECs) and causes extensive damage to the endothelium and the subjacent internal elastic lamina, then gains access to the media of affected vessels. Increased vascular permeability and leukocyte infiltration resulting from generation of chemotactic factors lead to hemorrhage and edema around these vessels.116,117 In addition to ECs, EAV also replicates well in macrophages in infected horses; EAV infection of cultured equine ECs and macrophages leads to their activation, with increased transcription of genes encoding proinflammatory mediators, including interleukin-1 beta (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α).118 Furthermore, virulent and avirulent strains of EAV induced different quantities of TNF-α and other proinflammatory cytokines from both infected ECs and macrophages. These studies strongly suggest that cytokine mediators that are produced by ECs and macrophages have a central role in the pathogenesis of EVA.
Evidence suggests that abortion after EAV infection of pregnant mares is the result of a lethal fetal infection rather than myometritis or placental damage that impairs progesterone synthesis, leading to fetal expulsion.109 The tissues of aborted fetuses contain higher titers of virus than those of the dams from which they abort, indicating that substantial virus replication occurs in the fetus itself.109 The stress that results from fetal infection would be expected to activate the fetal hypothalamic-pituitary axis, thus inducing abortion.
CLINICAL FINDINGS
The clinical severity of equine arteritis virus (EAV) infection of horses varies greatly.1,58,61,96 The vast majority of EAV infections are inapparent, especially those that occur in mares bred to persistently infected stallions.1,65,92 Outbreaks of clinical disease caused by EAV infection, equine viral arteritis (EVA), are characterized by one more of the following: abortion of pregnant mares; fulminant infection of neonates, leading to severe interstitial pneumonia or enteritis; and systemic illness in adult horses, with any combination of leukopenia and pyrexia, respiratory signs with nasal and ocular discharge, peripheral edema, hives, and persistent infection of stallions. The clinical signs observed in natural cases of EVA vary considerably among individual horses and between outbreaks and depend on factors such as the age and physical condition of the horse(s), challenge dose and route of infection, strain of virus, and environmental conditions.1,96 Although there is only one serotype of EAV, the clinical disease produced by different virus strains ranges from severe, lethal infection caused by the horse-adapted Bucyrus strain to clinically inapparent infection.20,66,109 Very young, old, debilitated, and immunosuppressed horses are predisposed to severe EVA.
Regardless of the infecting virus strain, the vast majority of naturally infected horses recover uneventfully from EVA. Young foals, however, can develop fatal infections that lead to severe, fulminating interstitial pneumonia,96,98 and foals up to few months of age can develop a rapidly progressive “pneumoenteritis” syndrome. With the notable exceptions of abortion and fulminant respiratory disease in foals, mortality rarely if ever occurs in natural outbreaks of EAV. The highly virulent horse-adapted Bucyrus strain of EAV (which causes high mortality in healthy adult horses) is not representative of field strains of the virus and is best regarded as an aberration.
Clinical cases of EVA are characterized by an incubation period of 2 to 14 days (6-8 days after venereal exposure), pyrexia of up to 41° C that may persist for 2 to 9 days (Fig. 14-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree