Chapter 33 Enteral and Parenteral Nutrition
Nutritional support is aimed at minimizing development of malnutrition in animals at risk, while maintaining or enhancing immunologic and intestinal barrier function. The specific indications for enteral nutrition in people have changed over the years, and it is no longer contraindicated in patients with pancreatitis, ileus, and intestinal dysmotility.1 Many techniques for obtaining enteral access are available, and the approach depends on several variables, including anticipated duration of enteral support, risk of aspiration, integrity of the gastrointestinal tract, the animal’s temperament, the clinician’s expertise, and the animal’s tolerance of anesthesia.
Rationale for Enteral Nutritional Support
Enteral feeding is indicated for animals unable to ingest adequate amounts of calories, but that have sufficient gastrointestinal function to allow digestion and absorption of feeding solutions delivered into the gastrointestinal tract via an enteral feeding device. The rationale for prescribing enteral nutrition rather than parenteral nutrition (PN) is based on the superior maintenance of intestinal structure and function,2 reduced infection rates,3 and reduced cost of enteral alimentation. The average daily cost of total parenteral nutrition (TPN), hereafter referred to as central parenteral nutrition (CPN), for maintaining the caloric requirements of a 20-kg dog at the University of California, Davis, Veterinary Medical Teaching Hospital, is approximately five to 30 times greater (excluding catheter costs) than the cost of a commercial liquid enteral formula, and 60 times greater than the cost of a commercial canned diet for intestinal disorders. The most important stimulus for mucosal cell proliferation is the direct presence of nutrients in the intestinal lumen.4 Bowel rest as a consequence of starvation or administration of CPN leads to villous atrophy,5 increased intestinal permeability, and a reduction in intestinal disaccharidase activities.6 Prolonged fasting in the stressed, critically ill animal can lead to intestinal barrier failure and increased permeability to bacteria and endotoxins. However, enteral nutrition may have shortcomings including underfeeding, perceived intolerance, aspiration, access-related complications, and diarrhea.7
Patient Selection for Nutritional Support
Efforts to assess nutritional status and attempts to decide whether nutritional support is required on the basis of a single biochemical measurement or body weight determination are simplistic and of limited value. Objective methods of assessing nutritional status, such as body composition measurement (anthropometry, bioelectrical impedance measurements, dual-energy X-ray absorptiometry) are still in their infancy in clinical veterinary medicine, with the result that a subjective global assessment of an animal’s nutritional status needs to be performed. This technique is based on easily collected historical information (changes in oral intake, degree of weight loss, presence of vomiting or diarrhea) and changes found on physical examination (muscle wasting, body condition, and presence of edema or ascites). A technique referred to as subjective global assessment was developed for the nutritional assessment of human patients approximately 30 years ago.8 The technique relies upon readily available historical and physical parameters to identify malnourished patients who are at increased risk for complications and who would benefit from nutritional intervention. The assessment involves determining (a) whether nutrient assimilation has been restricted because of reduced food intake, maldigestion, or malabsorption; (b) whether any effects of malnutrition on organ function and body composition are evident; and (c) whether the patient’s disease process influences its nutrient requirements. The subjective global assessment can be adopted to dogs and cats by determining the following five aspects of the animal’s medical history: (a) weight loss, (b) voluntary food intake, (c) the presence of persistent gastrointestinal signs, (d) the animal’s functional capacity (e.g., presence of severe weakness or exercise intolerance), and (e) the metabolic demands of the patient’s underlying disease state.
The body weight of the animal cannot be equated with its state of nourishment because it does not differentiate between fat, lean tissue, and extracellular water. The animal’s serum albumin concentration and total lymphocyte count are insensitive determinants of nutritional status because of the large number of disease processes that influence these parameters unrelated to the effects of malnutrition (see Chapter 30). Nutritional support should be considered for animals that demonstrate recent unintentional weight loss that exceeds 10% of optimal body weight or for those whose oral intake has been or will be interrupted for more than 5 days. Animals with increased nutrient losses from chronic diarrhea or vomiting, wounds, renal disease, or burns should also be considered for nutritional support. Numerous methods exist for quantifying body composition and body fat mass in companion animals. In a clinical setting, the most widely accepted and practical method of body condition evaluation is condition scoring using visual assessment and palpation. This method affords a reproducible and clinically useful assessment of nutritional status. The body condition score is not affected by fluid shifts that can readily impact body weight, facilitating the improved assessment of nutritional status in the hospital or intensive care environment. The most widely accepted system is the nine-integer scale system, which correlates well with body fat mass determined by dual-energy X-ray absorptiometry.9 Body condition scoring schemes do not incorporate the loss of lean body tissue, although the recent implementation of a muscle condition score could further enhance the assessment of nutritional status10 (see Chapter 30).
Calculation of Nutritional Requirements
Nutritional support provides substrates for gluconeogenesis and protein synthesis11 and provides the energy needed to meet the additional demands of host defense, wound repair, and cell division and growth. The anticipated duration of nutritional support should be determined and factored into the nutritional support plan. In addition, the most optimal route of nutritional support should be determined (enteral versus parenteral) based on the underlying disease process, the integrity of the gastrointestinal tract, and the patient’s clinical signs (intractable vomiting or diarrhea, dysphagia, etc.).
The provision of nutritional support should provide sufficient substrates for gluconeogenesis, protein synthesis, and energy to sustain vital physiologic processes such as immune function, wound repair, and cell division.12 Although energy expenditure has been determined using indirect calorimetry in research populations of dogs, utilization of mathematical formulas remains the most efficient, cost-effective, and practical means of estimating a patient’s energy requirement. An estimate of an animal’s resting energy requirement (RER) is needed to determine the minimum amount of food necessary to sustain critical physiologic processes. The RER is the animal’s energy requirement at rest in a thermoneutral environment and in a postabsorptive state. A linear formula can be applied to determine the RER of dogs and cats weighing between 2 and 45 kg, or, alternatively, an allometric formula (preferred by the author) can be applied to dogs and cats of all weights.
Allometric formula: RER (kcal/day) = 70 (body weight in kg)0.75
Linear formula: RER (kcal/day) = 30 (body weight in kg) + 70
Until recently, many clinicians multiplied the RER by an “illness factor” between 1.1 and 2 to account for the increased metabolism associated with different disease states and injuries. Veterinary nutritionists discourage the implementation of this extrapolated and subjective practice, and advocate a more cautious approach to minimize overfeeding and potential metabolic complications such as refeeding syndrome.13 Nutritional support should initially deliver sufficient calories and protein to meet the patient’s RER at its current weight, adjusted for body condition. Close observation of changes in body weight, physical examination findings (decreased subcutaneous fat stores, muscle wasting, and presence of edema or ascites), and ongoing losses (diarrhea, vomiting, exudative wounds) will help determine whether to increase or decrease the patient’s caloric intake.
Diet Selection
A variety of disease-specific enteral formulas are available for the management of critically ill human patients with renal disease, liver disease, diabetes mellitus, chronic obstructive pulmonary disease, and food allergies. The hepatic formulas offer increased amounts of branched chain amino acids: valine, leucine, and isoleucine; and reduced amounts of aromatic amino acids: phenylalanine, tyrosine, and tryptophan, compared with standard products. These alterations purportedly promote a reduced uptake of aromatic amino acids at the blood–brain barrier, reducing the synthesis of false neurotransmitters, and thereby ameliorating the neurologic symptoms that occur with hepatic encephalopathy (refer to Chapters 17 and 61).14,15 Evidence supporting the use of hepatic formulas is very limited and controversial, and there is currently no clear consensus supporting the use of branched chain amino acids supplementation in people with hepatic cirrhosis and encephalopathy.14,15 Enteral formulas enriched with arginine, omega-3 fatty acids, glutamine, and nucleotides are considered to enhance the immune response, although a systematic review of the evidence by Heyland et al. showed that immunonutrition reduced septic complications in critically ill adults, but this reduction did not result in reduced mortality.16
Commercial blended pet food diets are recommended for feeding via esophagostomy or gastrostomy tubes. In select cases, the feeding of a liquid enteral formulation may be indicated for feeding via nasoesophageal or jejunostomy tube. Feeding should be delayed for 12 to 24 hours after placement of a gastrostomy tube, to allow return of gastric motility and allow formation of a fibrin seal. In contrast, feeding can be instituted immediately after esophagostomy tube placement, once the animal has fully recovered from anesthesia. Diet can be administered as bolus feedings or continuous infusion when a gastrostomy tube is used for feeding. Improved weight gain and decreased gastroesophageal reflux have been reported in human patients given continuous feedings,17 although similar studies are lacking in the veterinary literature. If continuous feeding is employed, it should be interrupted every 8 hours to determine the residual volume by application of suction to the feeding tube. If the residual volume is more than twice the volume infused in 1 hour, feeding should be discontinued for 2 hours, and the rate of infusion decreased by 25% to prevent vomiting. Treatment with metoclopramide (1 to 2 mg/kg/24 h as a constant-rate infusion) may be used to enhance gastric emptying and decrease vomiting.18
With bolus feeding, the required daily volume of food should be divided into four to six feedings. Dogs and cats are usually fed approximately 25% of their caloric requirement on the first day of feeding, with a gradual increase of 25% of the caloric requirement per day. Most animals are able to reach their energy requirement by the fifth or sixth day of feeding. The food should be warmed to room temperature and fed slowly through the tube to prevent vomiting. Flushing the tube with 15 to 20 mL of lukewarm water helps prevent clogging. Before each feeding, the tube should be aspirated with an empty syringe to check for residual food left in the stomach from the previous feeding. If more than half of the last feeding is removed from the stomach, the feeding should be skipped and residual volume rechecked at the next feeding. Jejunal feeding can be started within 6 hours of tube placement if peristalsis is present. Continuous feeding should be used with jejunostomy feeding to avoid abdominal cramping and diarrhea associated with bolus feeding via this route. Continuous infusion is recommended at an initial flow rate of 1 mL/kg/h and increased gradually over 48 hours until the total daily volume can be given over a 12- to 18-hour period.19
Enteral Feeding Access Devices
Most feeding tubes today are made of polyurethane or silicone. The main shortcoming of silicone is related to its stiffness and flexibility. Silicone feeding tubes require thick sidewalls to obtain tube-wall integrity or stiffness. Because of this, the internal diameter of a silicon feeding tube is smaller than the internal diameter of a similar-sized polyurethane tube that does not require the degree of sidewall thickness for tube integrity.20 The flexibility and decreased internal diameter of silicone tubes may lead to clogging or kinking of the tube. In addition, silicone is known for notch sensitivity that is associated with propagation of a defect in the material when the silicone gets a nick or a tear in it.20 New feeding tube materials are being developed that are copolymers of silicone and polyurethane and other polymer end groups in an effort to mimic the softness of silicone and the durability and wall thickness of polyurethane. The French unit measures the outer lumen diameter of a tube (each French unit is equal to 0.33 mm).
Nasoesophageal Tubes
Nasoesophageal tubes are a simple and efficient choice for the short-term (less than 10 days) nutritional support of most anorectic hospitalized animals that have a normal nasal cavity, pharynx, esophagus, and stomach.21 Nasoesophageal tube feeding is contraindicated in animals that are vomiting, comatose, or lack a gag reflex. Polyvinylchloride (Infant Feeding Tube, Argyle Division of Sherwood Medical, St. Louis, MO) or red rubber tubes (Robinson catheter, Sherwood Medical, St. Louis, MO) are the least-expensive tubes for dogs and cats, although the polyvinylchloride tubes may harden within 2 weeks of insertion and cause irritation or ulceration of the pharynx or esophagus. Although tubes made of polyurethane (MILA International, Inc., Erlanger, KY) or silicone (Global Veterinary Products, Inc., New Buffalo, MI) are more expensive, they are less irritating and more resistant to gastric acid, allowing prolonged usage. An 8-French, 91-cm tube with or without a tungsten-weighted tip is suitable for dogs weighing more than 15 kg. A 5-French tube is more comfortable for cats and smaller dogs.
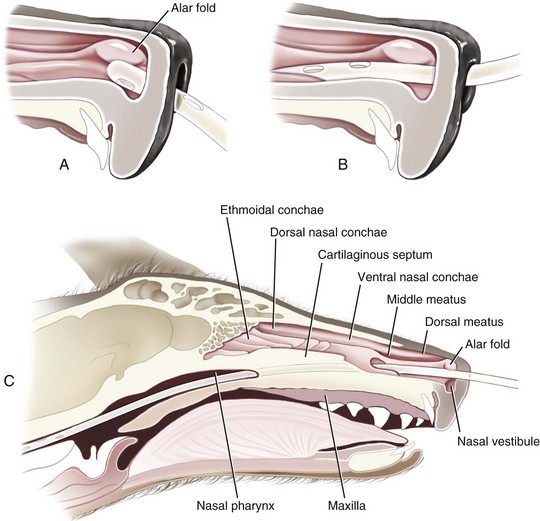
The tube should terminate in the distal esophagus to decrease the likelihood of reflux esophagitis,22 and this is facilitated by ensuring that the length of the tube approximates the distance from the tip of the nose to the seventh or eighth intercostal space. A tape marker is placed on the tube once the appropriate measurement has been made. Desensitization of the nasal cavity with 0.5 to 1 mL of 0.5% proparacaine hydrochloride is recommended. The head is tilted upward to encourage the local anesthetic to coat the nasal mucosa. The tip of the tube is lubricated with 5% lidocaine viscous prior to passage. The tube is passed by maintaining the animal’s head in the normal angle of articulation (avoid hyperflexion or overextension of the head and neck) and gently directing the tip of the tube in a caudoventral medial direction. The tube should move with minimal resistance through the ventral meatus and nasopharynx and into the esophagus. Nasoesophageal intubation is more difficult to perform in dogs because of their long, narrow nasal passages and extensive turbinate structures. In addition, the presence of a small ventral ridge at the proximal end of the nasal passage in dogs necessitates directing the tip of the tube dorsally initially to allow passage over the ventral ridge and into the nasal vestibule (Fig. 33-1).21 The tube is then directed in a caudoventral and medial direction while pushing the external nares dorsally.23 This maneuver opens the ventral meatus and guides the tube into the oropharynx.
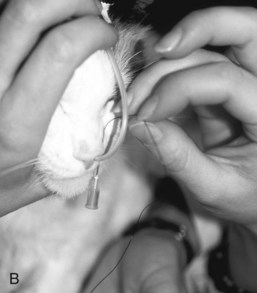
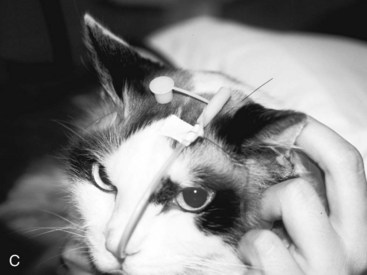
If the tube is unable to be passed with minimal resistance into the oropharynx, it should be withdrawn and redirected because it could be positioned in the middle meatus with the tip encountering the ethmoid turbinate. Once the tube has been passed to the level of the tube marker, it should be secured as close to the nostril as possible, with either suture material (Fig. 33-2A) or glue (Superglue, Loctite Corp., Cleveland, OH), although glue can cause a dermatitis in some animals. A second tape tab should be secured to the skin on the dorsal midline between the eyes (Fig. 33-2B and C). In the cat, the tube must not exit laterally nor come in contact with the whiskers. An Elizabethan collar is usually required for dogs to prevent inadvertent tube removal; most cats, however, do not require such a device. Removal of the tube is facilitated by clipping the hair that is attached to the glue.
After placement, the tube position is checked by injecting 5 to 10 mL of air while ausculting the cranial abdomen for borborygmus, by infusing 3 to 5 mL of sterile saline or water through the tube and observing for a cough response21 (although a lack of a cough response is not always a reliable indicator of tube placement), or by obtaining a lateral survey thoracic radiograph. Verification of placement can also be done with an end-tidal CO2 monitor. Tubes placed within the esophagus or stomach should yield no CO2 when checked with an end-tidal CO2 monitor. The most common complications associated with the use of nasoesophageal tubes include epistaxis, dacryocystitis, rhinitis, tracheal intubation and secondary pneumonia, and vomiting.21
Esophagostomy Tubes
The patient should be placed in right lateral recumbency, and the left lateral cervical region clipped and aseptically prepared for tube placement.24–26 Placement of the feeding tube in the left side of the neck is preferred because the esophagus lies slightly left of midline making left-sided placement more desirable. A 14- to 20-French red rubber catheter (Robinson catheter, Sherwood Medical, St. Louis, MO), silicone catheter (Global Veterinary Products, Inc., New Buffalo, MI), or polyurethane catheter (MILA International, Inc., Florence, KY) should be premeasured from the midcervical esophagus to the seventh or eighth intercostal space, and marked with a permanent marker to ensure the distal end of the catheter terminates in the distal esophagus.22 The left midcervical area is aseptically prepared from the angle of the mandible to the thoracic inlet. Three basic techniques for placement of a midcervical esophagostomy tube have been described.24–26
Percutaneous Feeding Tube Applicator Technique
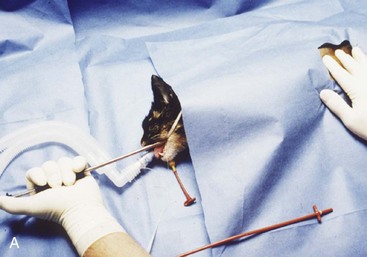
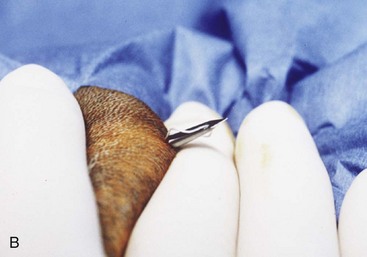
An alternative tube esophagostomy technique utilizing an ELD percutaneous feeding tube applicator or similar device can be used.8 The applicator is inserted into the midcervical esophagus via the oral cavity. The distal tip is palpated, and an incision is made through the skin and subcutaneous tissue over the tip of the ELD. Activate the spring-loaded instrument (Fig. 33-3A) to advance the trocar through the esophageal wall and incision (Fig. 33-3B) The distal end of the feeding tube is secured to the eyelet of the trocar with suture material. The ELD device and attached feeding tube are retracted into the esophagus and exteriorized out of the oral cavity. The feeding tube is redirected into the midcervical esophagus after inserting a wire stylet into the distal tip of the feeding tube. The tube is secured to the skin as mentioned above previously.
Percutaneous Needle Catheter Technique
This method incorporates the use of an esophagostomy introduction tube (Van Noort esophagostomy tube set, Global Veterinary Products, Inc., New Buffalo, MI) (Fig. 33-4) that is introduced into the midcervical esophageal area. The slot in the distal portion of the tube is palpated, and a Peel-away sheath needle (Global Veterinary Products, Inc., New Buffalo, MI) is introduced into the distal portion of the tube. The needle is removed from the sheath, and a 10-French catheter is introduced through the sheath to the distal third of the esophagus. The sheath is peeled away and the esophagostomy tube is carefully removed. The feeding tube is secured as described previously. This technique has limitations as the small diameter of the feeding tube (10 French) only allows for the administration of fluids and liquid enteral formulas.
Despite the potential for esophageal scarring and stricture formation, esophageal stricture or a persistent esophagocutaneous fistula have not been reported. The most common minor complication is peristomal inflammation, with peristomal abscessation occurring infrequently.24–27 Most of the inflammatory reactions are mild and respond to thorough cleansing with topical antibiotics. Other less-common complications include vomiting of the tube into the oral cavity and tube obstruction.24–27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree