Disorders of the Skin
Normal Hair and Skin
Llama hair contains both thick primary guard hairs and finer, shorter secondary hairs in an approximately 1 : 9 ratio.1 The primary hairs are rarer or absent in finer-fleeced alpacas.2 In both species, hair is often medullated. In thick-fleeced areas, hair follicles are oriented obliquely to the surface of the skin, probably to provide an insulating mat of hair. In the thinner-fleeced areas, hair follicles are perpendicular. A mix of simple and compound follicles is present. Compound follicles occur most commonly in areas of dense hair coat and usually consist of 3 to 9 secondary fibers and 0 to 2 primary hairs. Functionally, primary hairs are thought to shed water and protect the underlying secondary hairs and skin from injury. The secondary hairs act as insulation against both heat and cold.
Unlike Old World camelids or wild New World camelids, llamas and alpacas have a variety of possible hair colors. These may be important in disease, as white-haired areas appear most sensitive to solar damage and develop early lesions with photosensitization, whereas dark-fleeced areas are thought to be more vulnerable to skin diseases in general.3 Eyelids are often pigmented, even in animals with white head hair.
The skin itself is thickest on the neck and dorsal thorax and generally becomes thinner in more ventral locations in llamas.1 In alpacas, it is thickest on the neck, pasterns, and interdigital spaces.2 Neck skin of intact male or late-castrated llamas is especially thick as a defense against bites during dominance fighting and is two to three times thicker than in other areas. Exceptions to the trend toward ventral thinning are the thick areas along the mucocutaneous junctions, the metatarsal and interdigital “scent” glands, and the foot pads, as well as the thin skin of the ear pinnae. The metatarsal scent glands actually have little glandular tissue and are more likely to be vestigial digits than an important site of pheromone production. The average skin thickness has been reported to be 3.9 millimeters (mm), as in horses and camels, thicker than in small ruminants, and 65% the thickness of cattle skin. Increasing thickness is usually from increasing width of the dermis.
Heavily haired areas in camelids appear to have fewer sebaceous glands compared with ovine skin. This results in a lack of lanolin on camelid fiber, which may affect how topical medications distribute over the fiber. Sweat glands are present in similar frequencies to other species. Camels are reported to have fewer sweat glands to prevent water loss, but that has not been confirmed in New World camelids.4
Camelid skin has a number of specialized areas. These include the foot pads, metatarsal scent glands (“Chestnuts”), interdigital scent glands, teats, external ear pinnae and canals, and toe nails. The nails are similar in structure to the hooves of horses or cattle, except that they are non–weight bearing. The foot pads have the thickest epidermis of all skin, up to around 2 mm.1
Heavily Fleeced Areas
The heavily fleeced areas of camelids are infrequently affected. The most common diagnosis is pediculosis, which, ironically, has received little attention in the scientific literature.5–8 Affected camelids show signs of pruritus, including restlessness, scratching behavior, patchy traumatic fiber loss, excoriation, dry scale, and erythema, particularly on the neck, tailhead, and flanks. In alpacas, the head and ears may be affected as well. With excoriation, some moist discharge and matting of the fiber may occur. Sucking lice, which feed on lymph and blood rather than surface debris, may induce anemia. Severely affected animals with any sort of louse infestation will appear generally unthrifty.
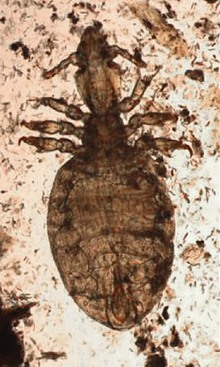
Camelids are affected by both sucking (Microthoracius spp.; Figure 35-1) and chewing (or biting; Bovicola [Damalinia] breviceps; Figure 35-2) lice, chewing lice being more common in most areas. Mixed infections have been seen.3 Clinical infestation is most common during the colder months, partially because of the long fleeces during that period as well as possible confinement housing or huddling facilitating transmission. Other times of close animal contact or commingling of animals such as shows or sales, night confinement, breeding or nursing, or grouped animal transport also promote direct spread of lice. Shared coats, brushes, or shearing equipment may act as vectors as well. Lice have poor environmental hardiness, especially in winter, so transmission usually requires fairly direct contact between animals. Diagnosis is achieved by finding the lice or nits in the fleece. Biting lice are 1 to 2 mm long and are usually easy to find by parting the fleece at regular intervals near the affected area. Sucking lice are up to 3 mm long. Nits of both types are around 1 mm long and attach to hair shafts 5 to 15 mm from the skin. The type of louse may be identified by microscopic or hand lens examination of the head of the louse, which is almost as wide as the abdomen in chewing lice, and about one quarter the width of the abdomen in sucking lice. Dead lice are also occasionally found on examination of feces, evidence that the animal is chewing on its own irritated skin.

Figure 35-2 Ventral aspect of Bovicola [Damalinia] breviceps, the chewing louse. Note the broad head and thorax, and round mouth.
Shearing and exposure to sunlight and fluctuating temperatures are very effective at reducing lice numbers but may be impractical during certain times of the year. Oral or parenteral avermectin at 1.5 to 2 times labeled ruminant doses, or similar compounds, may be effective against sucking lice but are thought to be less efficacious against chewing lice.7 Topical livestock antilouse dips, sprays, and powders are effective against both types, with pyrethrin or permethrin (cyfluthrin or others) appearing to be useful and safer compared with cholinesterase inhibitors such as organophosphates or carbamates. For topical, nonabsorbed medications, label directions for cattle or small ruminants are followed. Complete coverage of the affected areas is desired. Sprays or solutions may be daubed or “painted” onto sensitive areas such as the face. Pour-on type medications may not always distribute sufficiently, and data to determine their efficacy are insufficient. α-Adrenergic agonists (0.025 to 0.05% amitraz [5 to 10 milliliter per liter [mL/L] of 5% solution]) and 0.0025% spinosad (1 : 1000 dilution of 25 grams per liter [g/L] stock solution in water) also containing 0.1% alcohol alkylate (also 1 : 1000 dilution of stock solution) as a wetting agent, using approximately 8 L of final solution per alpaca) have also been used successfully.5 Shearing may be necessary to allow for adequate contact of topical agents. Treatments should be repeated every 7 to 14 days to account for larvae hatched after the previous treatment until lice are no longer found. All contact camelids should be treated, if possible.
Dermatophilosis (“rain rot”) may be seen, particularly in areas with a wet, mild climate or in dry areas with heavy seasonal moisture. It is a well-known disease in camels, but scientific reports of disease in New World camelids are rare.9–11 The causative organism Dermatophilus congolensis is a gram-positive coccoid organism, which grows in filaments. It releases zoospores that are motile and infective under wet conditions but also may develop into a longer-lived, capsulated form that survives up to several years under dry conditions. It is principally maintained within chronic skin lesions on carrier animals. Isolates from camels have been successfully transplanted to cattle and sheep, suggesting that a variety of species could act as reservoirs.9 Environmental contamination may also play a role. Moisture leads to reactivation.
The motile form is thought to require a skin break or irritation to allow it to colonize the host animal. Hence, other insults, including ectoparasites, shearing injuries, and penetrating thorns or awns, are thought to promote invasion and spread within a herd. In camels, tabanid flies are thought to be important in the transmission and establishment of the original skin injury.9 Host immunity appears to be a factor as well, and in both Old and New World camelids, yearlings and younger animals appear most prone to developing diffuse or deep infections.
A different syndrome of deep infection has been identified in Australia and seen sporadically elsewhere.12 Affected camelids may have mild superficial dermatitis but, more strikingly, have focal subcutaneous swellings. These appear to be most common on the exposed, lightly fleeced areas such as the face, neck, distal extremities, and inguinal region. The swellings are the result of local abscesses and granulomas accompanied by regional lymphadenopathy. The role of D. congolensis may be established by bacteriologic culture or histopathologic examination of a lesion or its contents. Other organisms, including bacteria and fungi, may be isolated as well. Abscesses, which are filled with caseous material, may contain evidence of plant material, suggesting that penetrating or migrating plant particles are responsible for the deep infection.
Treatment of the superficial form includes use of parenteral antibiotics (usually penicillin, ceftiofur, or oxytetracycline for 4 to 6 days at standard dose rates, longer in generalized or refractory cases), coupled with protection from the wet environment, local clipping and crust removal, and daily cleansing of lesions with topical disinfectants for up to 10 days. Streptomycin, alone or in combination with penicillin, may be effective in countries where it is available. Sulfa antibiotics have poor efficacy. The deep form rarely responds satisfactorily to short courses of antibiotics, and extended courses have not been adequately tested. Local abscesses may respond to surgical excision or drainage, but lesions are often multiple and extensive, and complete resolution is unlikely. Abscesses have recurred or developed at other locations in alpacas after excision.12
A variety of tumors may occur on the fully fleeced skin, but overall prevalence is rare. Squamous cell carcinoma and fibroma or fibropapilloma-type lesions are much more common on less protected skin, presumably caused by sunlight, injury, infection, or other irritants that promote those tumors. Metastatic tumors are possible as well.13 Trichoepithelioma is seen sporadically, mainly in older animals, including one long-term member of the teaching herd in Oregon State University.14,15 Multiple hybrid follicular cysts may be present, particularly over the dorsum, the flanks, and, occasionally, the neck. Most appear as unpigmented or lightly pigmented, slightly soft nodules 1 to 2 cm in diameter. Some become quite large. They do not appear inflamed or painful. Affected animals appear otherwise thrifty, although masses may be damaged and bleed during shearing. Diagnosis may be achieved by excisional biopsy. Smaller samples are less diagnostic because they may by filled with cyst contents, which are usually waxy or caseous and may contain fragments of coiled hair fibers. Treatment is rarely necessary but could include excision of the troublesome masses.
Lightly Fleeced Areas
The lightly haired areas are not as protected compared with the fleeced areas and hence appear to be much more vulnerable to trauma, infection, solar radiation, and ectoparasitism. Several authors agree that dark-haired camelids appear to develop more skin disease in this area of the body compared with light-fleeced herdmates.3,16
Bacterial folliculitis may lead to erythema and patchy, crusty, exudative hair loss, usually with minimal to mild pruritus. Papules, pustules, yellowish crusts, and epidermal collarettes are characteristic and help distinguish folliculitis from other lesions. Common sites are the head, the back, the ventrum, and the distal extremities. Occasionally, a predisposing factor such as insect bites, contact dermatitis, intertrigo, immunodeficiency, or debilitation exists, some of which increase the level of pruritus.15 In severe cases, folliculitis may progress to cellulitis. Diagnosis is by culture, preferably by aspiration from a nonruptured pustule or from a biopsy sample. Staphylococcus intermedius is the most common isolate. Cytologic examination or biopsy additionally reveals bacteria, usually cocci, and a neutrophilic infiltrate. One study suggested that bacterial folliculitis is common.15 Failure to systematically examine cytologic preparations or perform bacteriologic cultures may lead to underappreciation and misdiagnosis. Affected areas should be scrubbed daily with an antiseptic solution or shampoo. Systemic antibiotics may be necessary as well. Choice of antibiotic should be dictated by culture results; common choices are penicillin, ceftiofur, or oxytetracycline for 7 to 21 days, typically continuing 7 days after clinical cure for superficial infections and 14 days for deep infections. Dermatophilosis (see above) may also affect the lightly fleeced regions with generalized infection or when insects or sharp objects damage the unprotected skin.
Dermatophytosis (ringworm) tends to affect the legs, the head, or the perineum. Winter confinement and shared feeders appear to increase risk. A variety of Trichophyton and Microsporum spp. have been reported, including T. verrucosum, T. mentagrophytes, M. nanum, and M. gypseum, all of which may cross species and some of which are zoonotic.3,17 Similar organisms cause ringworm in camels.18 Lesions tend to be multiple, focal, hyperkeratotic, nonpruritic, and alopecic patches, with thick crusts. However, crusts tend not to be as thick as the “asbestos-type” lesions seen in cattle, and the diagnosis may be missed without further testing. Diagnosis is achieved by examining plucked hair, a skin scrape sample, or a biopsy sample microscopically and identifying multiple arthroconidia surrounding, but not penetrating, hair shafts or by growing the organism in dermatophyte test medium. Because different dermatophyte species require different growing conditions, particularly T. verrucosum, multiple samples may be necessary. Even with a potassium hydroxide (KOH) preparation or special stains, the organisms may not be seen or may be hard to find on skin scrape or histopathology of a biopsy sample (Figure 35-3). Because of these diagnostic difficulties, the prevalence of dermatophytosis may be underestimated, and some empiric treatment may be indicated.
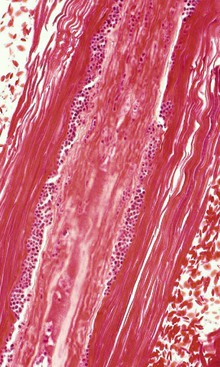
Figure 35-3 Punctuate ringworm arthroconidia (small, round, blue structures) colonizing an affected hair shaft.
A variety of mites affect the predominantly haired regions. These include the sarcoptic, chorioptic, and psoroptic mange mites, Demodex, the northern fowl mite, harvest mites, and possibly sheep keds. Mixed infections have been reported.19,20 Epidemiologic data are largely lacking, but one survey suggested that mange of all causes is most common during the summer months in England.21 Although regulations and enforcement vary geographically, it is important to acknowledge that in many areas, mange mites or, indeed, any transmissible infestation should be reported to the local health authorities. Show standards may also be in place to prevent the attendance of animals with transmissible disorders.
Of the mange mites, Sarcoptes scabei var. auchenidae is the most common in South America, where prior to the greater availability of avermectin, it was responsible for 95% of all illnesses caused by ectoparasites.22 Routine antiparasitic treatment improves productivity, with reduction in morbidity from mange a likely contributor to improvement.23 It burrows through all layers of the epidermis except for the basal layer, causing papular dermatitis primarily on the legs, axillae, inguinal region, and perineum.24 In severe cases, the infestation affects other regions such as the face or the flanks or becomes generalized (Figure 35-4). Pruritus may be moderate to intense, and scratching leads to erythema, alopecia, yellow crusting, skin thickening, bleeding, hyperpigmentation, and general ill thrift. Secondary bacterial infections may develop, and severe infestations may be fatal. The disease is known as Sarna in South America. It has been described in most countries where camelids are raised.3,19–22,25,26 However, it is uncommon in North America.

Figure 35-4 Severe alopecia, crusting, and self-trauma of the face of a llama with Sarna, Sarcoptes mange mite infestation. The llama had similar lesions over most of its body. (Photo courtesy of Woodburn Veterinary Clinic, Woodburn, OR.)
As a deep burrower, Sarcoptes organisms spend almost their entire lives on the host, surviving a few days, at best, in the environment. They complete their entire lifecycle from egg to adult in 7 to 14 days. Disease is most common in cold, moist weather. Mites spread through direct contact, communal dust baths, shared equipment, or bedding. Severe weather, transport, breeding, or other periods of close animal contact promote spread. Multiple animals may be affected simultaneously, with some individuals showing much more severe disease compared with their herdmates. Temporary infections of humans or other animals is also possible.26
Diagnosis is achieved by identifying the mites or their eggs on deep skin scrape of affected areas. If mites are not seen immediately, mixing the sample with 10% KOH for up to 20 minutes on the slide helps clear some of the debris. Although mites are usually plentiful and can be identified with low power (40×) microscopy, only a few are found on some animals. Dead mites are also occasionally found on examination of feces, evidence that the animal is chewing on its own irritated skin. Adult females have rounded bodies, less than 0.5 mm in length and width, four pairs of legs, and suckers (pedicels) on long unsegmented stalks on the front pairs. Males and those in immature stages are smaller and may have fewer legs. Rear legs rarely extend beyond the body margin. Skin biopsy may also reveal mites. Other histologic abnormalities include epidermal hyperplasia and hyperkeratosis, acute and chronic inflammation, and eosinophilic infiltration.24 Dermal neutrophilic, monocytic, or eosinophilic inflammation and fibrosis may exist as well. Without confirmation of seeing the mites by one of these methods, a tentative diagnosis may be reached through a positive treatment response. Sarcoptic mange is reportable in many areas.
A variety of agents have been used to treat sarcoptic mange. Efficacy of some is difficult to judge because response is not immediate, and in some cases, mixed infections complicate interpretation of the treatment response. Topical avermectin appears to have poor efficacy, possibly because it distributes inadequately across the lightly haired skin, especially when the skin is severely inflamed or thickened.25,26 A single dose of long-acting ivermectin led to complete clearance of live mites but took between 15 and 30 days to do so at 0.4 milligrams per kilogram (mg/kg; route unknown) and between 30 and 45 days at 0.2 mg/kg.24 Two doses of subcutaneous ivermectin (0.2 mg/kg) 10 days apart cleared infection within 24 days.20 Similar results were achieved with 4 weekly doses.19 Although 0.2 mg/kg of subcutaneous ivermectin appears to be effective, higher doses (up to 0.4 mg/kg) have been advocated.19,20,27 Multiple weekly doses of topical eprinomectin (0.5 mg/kg) and a single dose of subcutaneous doramectin (0.2 mg/kg) were deemed ineffective but the subjects were not followed up as long as those in the previous study.25 Topical weekly 0.025% to 0.05% amitraz (5 to 10 mL/L of 5% solution) on lesions appeared to work in those alpacas, but the residual efficacy of avermectin may have contributed to it. Others have reported treatment failure with a variety of agents, supporting the need for good posttreatment surveillance to judge efficacy.28 Use of topical fipronil spray for Sarcoptes in camelids has not been reported. This agent is gaining popularity as an acaricide in camelids and is likely to be effective against Sarcoptes. Users should keep in mind that fipronil is classified by the U.S. Environmental Protection Agency (EPA) as a class C agent, that is, a possible carcinogen. With any topical acaricide, weekly use of topical keratolytic cleansing agents may remove surface debris and allow better penetration.
Systemic antibiotics may be indicated to avoid or treat secondary bacterial infections. Animals should ideally be quarantined during treatment, and all contact animals should be treated. The delay before complete response, up to 45 days, should also be considered when treating animals coming into a herd.
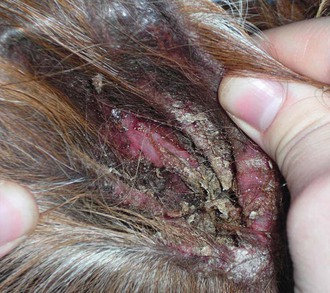
Psoroptes is less common and primarily affects the ears.15,20,22,29–32 Occasionally, mites are isolated from lesions in other areas more commonly associated with other mange mites. Clinical signs include head shaking; ear scratching; distracted behavior; erythema and thickening of the skin of the pinna and external ear canal; ceruminous crusting and purulent exudates within the ear; and, in worst cases, ear droop, head tilt, and ataxia (Figure 35-5). Secondary bacterial infections are common, and the development of neurologic signs indicates that the eardrum has ruptured and infection has spread to the middle or inner ear. Mites may be found by microscopic examination of deep skin scrapes or ear exudate swabs. Dead mites are also occasionally found on examination of feces, evidence that the animal is chewing on its own irritated skin. Adult mites are slightly larger than Sarcoptes, have longer legs that extend beyond the body, both front and back, and have terminal suckers (pedicels) extending from their front legs on long, jointed stalks (Figure 35-6). Some mites are occasionally found in clinically normal ears, which may protect reservoir populations. Additionally, survival off the host for as long as 84 days has been described.33 Parenteral avermectin is efficacious under most circumstances and has reduced, but not eliminated, Psoroptes populations outside of South America. Four to six weekly treatments may be required. However, cases still arise in populations regularly treated with avermectin. Additional treatments have included intraaural 0.25% fipronil spray or ivermectin (two drops diluted in saline per ear).29,31 Thorough cleaning of the ear before administration is likely to improve efficacy. Care must be taken not to use neurotoxic agents if perforation of the tympanic membrane has occurred. Secondary bacterial infections may resolve with successful mite treatment or may be treated with antibiotics. Otitis media or interna should be treated if suspected. (See Chapter 38). Treatment should be repeated weekly to biweekly for 2 to 4 times to eliminate mites newly hatched from eggs. Although direct animal contact is thought to be the most important means of transmission and environmental transmission to the ear is difficult to accomplish, the long off-host survival could dictate some environmental control measures as well. Psoroptic mange is reportable in many areas. Questions about transference of mites between camelids and other species persist.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree