CHAPTER 3 Development of the Nervous System
Malformation
NEURAL TUBE
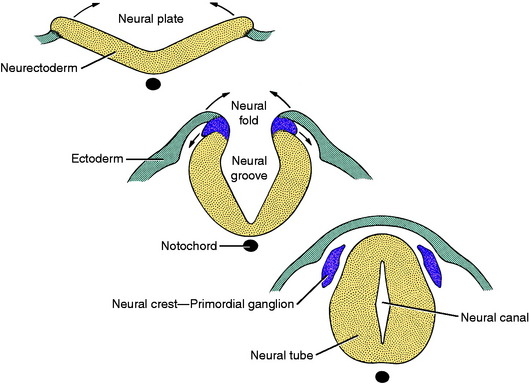
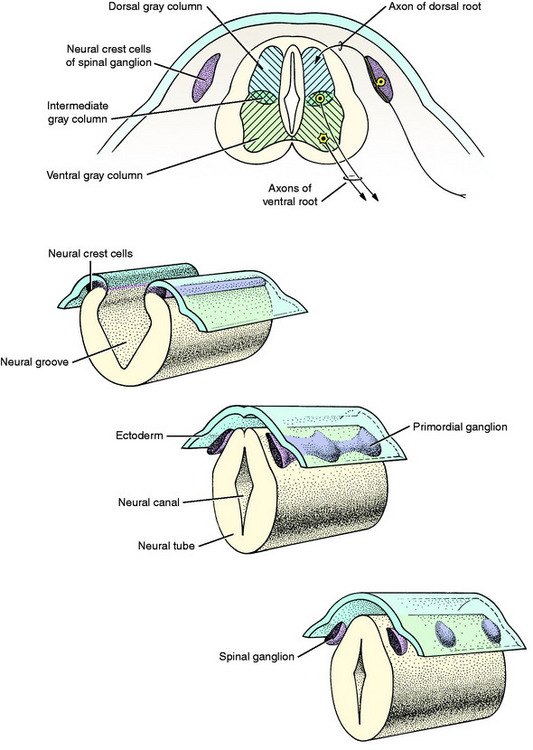
The central nervous system is a tubular structure originating from a proliferation of ectod ermal epithelial cells referred to as the neurectoderm, which is located dorsal to the notochord along the axis of the embryo. This thickened ectoderm, known as the neural plate, invaginates along this axis, forming a groove until the lateral extremities of the original plate, the neural folds, meet centrally and fuse over the neural groove to form a neural tube and canal. As the neural tube forms, it separates from the nonneural ectoderm which grows over the dorsum of the tube to fuse along the midline. As this fusion and separation of ectodermal layers occurs, a longitudinal column of ectodermal epithelial cells arises from the junction of nonneural and neural ectoderm and separates from these two structures when the neural tube is formed. These two bilateral columns, situated dorsolateral to the neural tube throughout its length, are the columns of neural crest cells (Fig. 3-1).
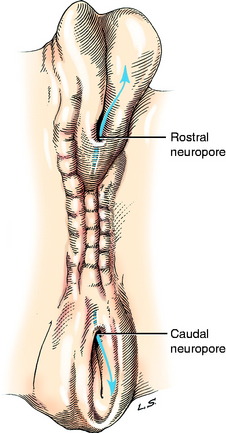
Closure of the neural tube progresses rostrally and caudally from the level of the site of development of the rhombencephalon, the most caudal division of the brain. The caudal closure forms the majority of the spinal cord. Closure of the brain portion of the neural tube may initially occur at multiple sites and progress rostrally and caudally. The locations of these sites vary among species of animals. Prior to complete closure, the most rostral opening is the rostral neuropore (Fig. 3-2). The caudal portion of the spinal cord develops from the caudal end of the closed neural tube as an extension of a column of neuroepithelial cells that grows caudally on the midline between the notochord and skin ectoderm. A cavitation of this column of cells produces an extension of the neural tube and its neural canal. This portion of the neural tube will ultimately form the caudal, the sacral, and a variable number of lumbar spinal cord segments. An opening may persist at the caudal end of the neural tube, allowing communication with the subarachnoid space of the leptomeninges at the conus medullaris.
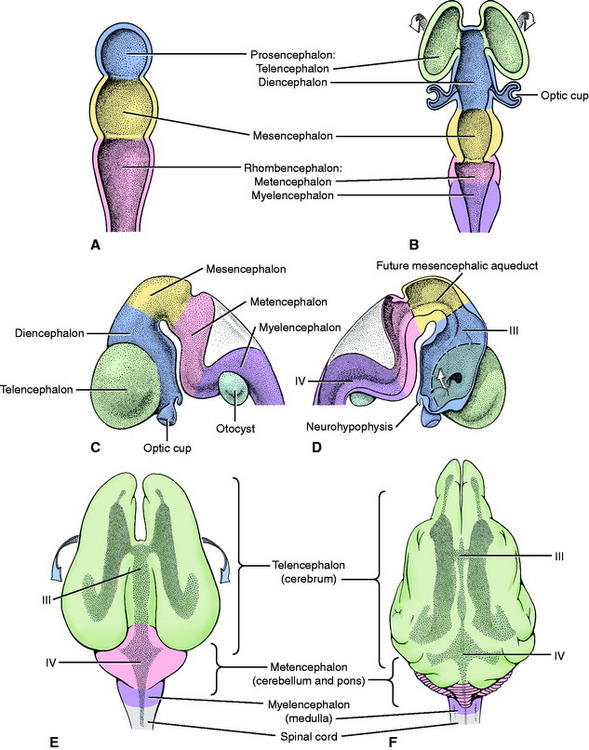
The rostral end of the neural tube develops rapidly and produces three vesicles, from rostral to caudal: the prosencephalon, mesencephalon, and rhombencephalon (Fig. 3-3). Early in its development the prosencephalon has lateral enlargements, the optic vesicles, which grow laterally to contact the overlying skin ectoderm. The further development of this primordial eye is described in Chapter 14, Visual System. Two additional swellings emerge from the rostral prosencephalon and grow out of the neural tube on each side laterally and dorsally. These telencephalic vesicles completely overgrow the original vesicular system and form the cerebral hemispheres. The portion of the prosencephalon that remains at the rostral end of the neural tube is the diencephalon. The optic vesicles remain associated with the diencephalon. The neural canal within the diencephalon is the third ventricle. It communicates rostrolaterally with the neural canal of each telencephalon (cerebral hemisphere), which is the lateral ventricle (first and second ventricles). This small communication on each side is the interventricular foramen. The nuclei of the thalamus and hypothalamus develop in the diencephalon. The neurohypophysis is a ventral outgrowth of the diencephalon. The cerebral cortex and basal nuclei develop in the telencephalon.
From the rostral rhombencephalon, the cerebellum or dorsal metencephalon develops dorsally. The remaining ventral metencephalon becomes the pons. The caudal rhombencephalon forms the myelencephalon or medulla oblongata. The fourth ventricle is the lumen of the neural canal in the rhombencephalon. It communicates with the meningeal spaces that develop around the neural tube by way of openings that arise in the wall of the neural tube caudal to the developing cerebellum. These openings are called the lateral apertures (see Figures 4-1 and 4-2). The neural canal continues caudally as the central canal of the spinal cord.
Cell Differentiation
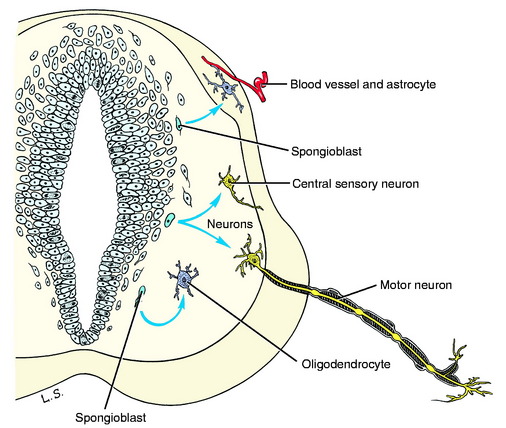
The cells that are differentiated are of two types: immature neurons and spongioblasts. Immature neurons are the primary parenchymal cells of the nervous system. They are often referred to as neuroblasts but this is a misnomer because once a neuron is formed it will not divide again as the term neuroblast implies. The differentiated immature neuron grows extensively, forming long processes in becoming a mature functioning cell, but it does not divide again. Spongioblasts are the progenitors of the neurectodermal supporting cells of the nervous system, the neuroglia (glue). Two of the three forms of glial cells are derived from these spongioblasts: astrocytes and oligodendrocytes (Fig. 3-4). The third glial cell is the microglial cell, which is mesodermal in origin. It is a monocyte that enters the nervous system from its blood supply.
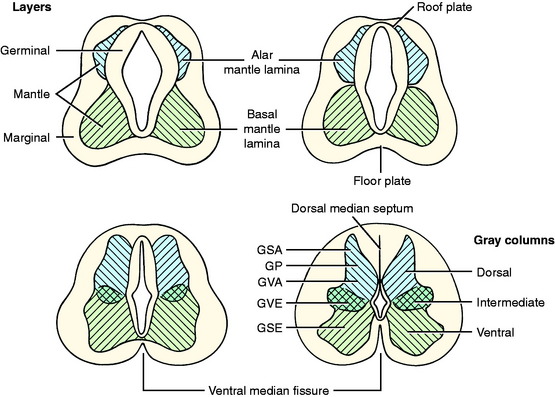
As the primitive neurons and spongioblasts are differentiated and grow and the neurons produce processes, the neural tube becomes arranged in three concentric layers (Fig. 3-5). Adjacent to the lumen of the neural tube is the germinal layer of proliferating neuroepithelial cells. This proliferative mitotic activity will ultimately be exhausted, reducing the germinal layer to a single layer of cells ranging from squamous to columnar and called ependymal cells. These ependymal cells line the entire lumen of the neural tube, which includes the ventricular system in the brain and the central canal of the spinal cord. Peripheral to this germinal layer in the embryonic neural tube is the thick layer of differentiated cells, the immature neurons, and spongioblasts. They form the mantle layer, which will ultimately become the gray matter of the definitive spinal cord, the nuclei of the brainstem, the nuclei and cortex of the cerebellum, and the basal nuclei and cerebral cortex of the telencephalon. The latter requires a migration of these neurons from the mantle layer to the external surface of the neural tube. The external layer of the neural tube is the marginal layer; it is composed primarily of the growing axonal processes of the neuronal cell bodies in the mantle layer. These axons will be myelinated by the oligodendroglial cells forming tracts in the white matter.
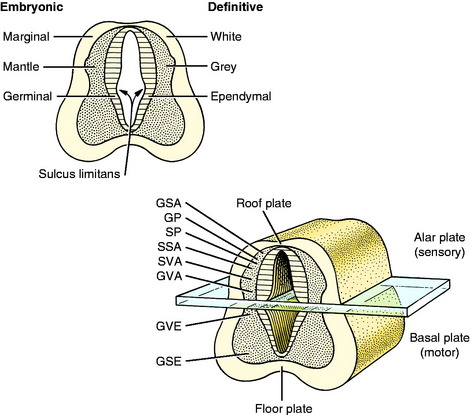
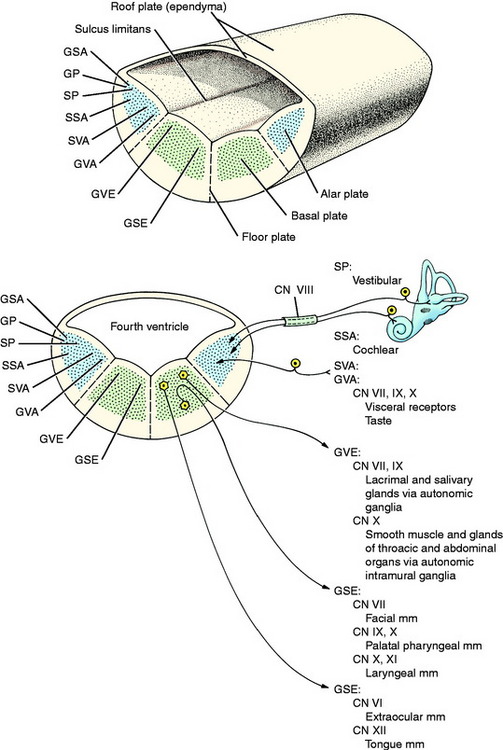
From the mesencephalon caudally, a longitudinal groove, the sulcus limitans, appears in the lateral wall of the neural canal. Thus the neural canal can be artificially divided into dorsal and ventral portions by an imaginary dorsal plane at the level of this sulcus. The dorsal portion is called the alar plate and the ventral portion the basal plate. Functionally the alar plate mantle layer is concerned predominantly with sensory systems, and the basal plate mantle layer with motor systems (see Fig. 3-5).
MEDULLA SPINALIS: SPINAL CORD (See Fig. 2-17)
In this text, the term spinal cord is used rather than medulla spinalis, the nomenclature preferred by Nomina Anatomica Veterinaria. The spinal cord provides the best example of the symmetric development of the neural tube by layers. Ventral growth of the two basal layers and associated marginal layers beyond the level of the floor plate (Fig. 3-6) leaves a separation between the two sides, which is the ventral median fissure. The mantle and marginal layers of the alar plates grow dorsally. The dorsal marginal layers fuse on the median plane to form a dorsal median septum that may be poorly defined. The external margin of this septum forms the dorsal median sulcus. This midline growth displaces the roof plate region ventrally, resulting in a reduction of the neural canal to form the small central canal of the spinal cord lined by ependymal cells. The mantle layer of the alar plate becomes the dorsal gray column (also referred to as horn), and that of the basal plate becomes the ventral gray column. The mantle zone at the plane of the sulcus limitans becomes the intermediate gray column (see Fig. 3-6).
The growth of axons of the basal plate neurons through the marginal layer and outside the neural tube forms the ventral root and part of the spinal nerve and further branching of the peripheral nerves. This includes the general somatic efferent neurons located in the ventral gray column and the general visceral efferent neurons located in the intermediate gray column adjacent to the sulcus limitans. These latter general visceral efferent (GVE) neurons are the preganglionic lower motor neurons of the autonomic nervous system. This intermediate gray column is only present in the thoracic, cranial lumbar and sacral spinal cord segments. In the other segments, it was present in the embryo but subsequently degenerated due to the absence of a target organ or biochemical attractant. These GVE neurons terminate in ganglia in the peripheral nervous system that contain cell bodies of the postganglionic neurons in this two-neuron lower motor neuron system (Figs. 3-7 and 3-8; see also Fig. 3-6).
Neural Crest
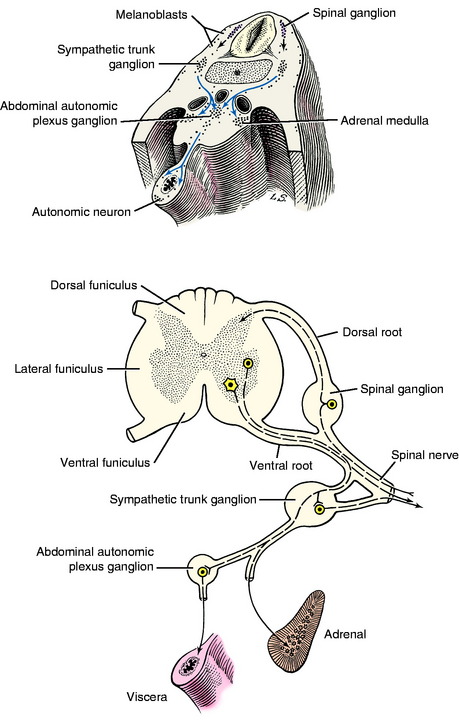
The neural crest cells are the cell bodies in the longitudinal column of cells that formed dorsolateral to the neural tube. Along the developing spinal cord segments, these cells provide the neurons that form the spinal ganglia at each segment. Adjacent to each somite a proliferation of neural crest cells forms the segmental spinal ganglion (see Figs. 3-1 and 3-7). One portion of the axon that emerges from each of these cell bodies grows centrally into the spinal cord segment to enter the alar plate dorsal gray column forming the dorsal root. The other portion of the axon grows distally to form a sensory component of the spinal nerve and further branches of the peripheral nerves. The point of penetration of the marginal white matter layer of the spinal cord segment by the axons in the dorsal and ventral roots divides the spinal cord white matter processes into three regions called funiculi. These are the dorsal, lateral, and ventral funiculi on each side of the spinal cord.
These latter cell bodies form the ganglia of the sympathetic trunk and the abdominal plexus sympathetic ganglia as well as the medullary cells in the adrenal gland (see Fig. 3-8). These adrenal medullary cells do not grow any processes but synthesize and elaborate into the blood stream the same endocrine substance, norepinephrine, that is the neurotransmitter released at the telodendron of the sympathetic postganglionic axon derived from the neural crest cells. Although the melanoblasts and GVE neurons seem unrelated, their common denominator is the unique metabolism of tyrosine, which provides melanin for the melanocytes and the norepinephrine for the neuron. In addition, there is extensive migration of the neural crest cells into the wall of the developing gastrointestinal tract. These will form the postganglionic neurons of the parasympathetic portion of the GVE lower motor neuron as well as interneurons; and they form the glial cells that develop in the wall of the bowel, creating what is referred to as the enteric nervous system. The latter is extremely extensive and complex and presumably is entirely derived from the neural crest cells. A similar migration forms the postganglionic parasympathetic neurons for the urogenital system. In addition to these nervous system structures, the neural crest contributes to the formation of bone and cartilage in the skull and derivatives of the branchial arches; to the wall of the great vessels at the base of the heart; and to thyroid parafollicular (C) cells, odontoblasts, a portion of the leptomeninges and the lemmocytes, Schwann cells, that form the myelin of the peripheral nervous system. This is an amazing display of developmental capabilities in an initial column of cells. When a student is asked about the origin of a structure, if there is any doubt, relying on neural crest is a worthwhile consideration!
MYELENCEPHALON: MEDULLA OBLONGATA (See Figure 2-11 through 2-16)
In this text, the term medulla oblongata is shortened to medulla. The medulla is the most caudal portion of the brainstem and is continuous caudally with the spinal cord. The basic formation of the medulla involves only a slight modification of the development described for the spinal cord. The narrow middorsal roof plate of the initial neural tube (see Figs. 3-5 and 3-6) is stretched extensively instead of being obliterated by the proliferating alar plate and marginal tissue as it is in the spinal cord. Imagine grasping the midline roof plate of the neural tube with both hands and then pulling your hands apart sideways. This would stretch out a thin layer of neural tube (roof plate) and displace the entire alar and basal plates to a lateral and ventral position (Fig. 3-9). This would enlarge the lumen of the neural tube to form the fourth ventricle of the medulla, which is covered dorsally by only a single cell layer of neuroepithelial cells. At this site these cells will not enter mitosis but will remain as a single layer of ependymal cells. The sulcus limitans that is present on the ventrolateral wall of the fourth ventricle provides the plane of division of the medulla into a ventromedial basal plate and a dorsolateral alar plate, which have the same functional significance as in the spinal cord development.
Throughout the brainstem, the mantle layer of the neural tube is broken up into nuclei that are collections of neuronal cell bodies with a common purpose, and they are interspersed with neuronal processes. Some nuclei are more distinct than others. The functional columns described in the spinal cord have a similar location in the brainstem. In addition, there are neurons in the medulla that are organized into functional groups that are present only in cranial nerves (see Fig. 3-9).
The sensory components of cranial nerves associated with the medulla arise primarily from primitive neurons that develop from the column of neural crest cells, with a few arising from ectodermal cells that proliferate from branchial arch ectoderm. The latter areas are referred to as cranial placodes. These two sources form the sensory ganglia of cranial nerves VII, IX, and X, which are concerned with general visceral afferent and special visceral afferent (taste) function. Ectodermal cells derived from the otic placode form the sensory ganglia of cranial nerve VIII, which is concerned with special proprioception for vestibular system function and with the special somatic afferent system for auditory function. These cranial nerve VIII ganglia are located in the inner ear within the petrosal portion of the temporal bone. Their axons course into the alar plate region of the medulla to synapse on cell bodies comparable to the dorsal gray column cell bodies in the spinal cord (see Fig. 3-9).
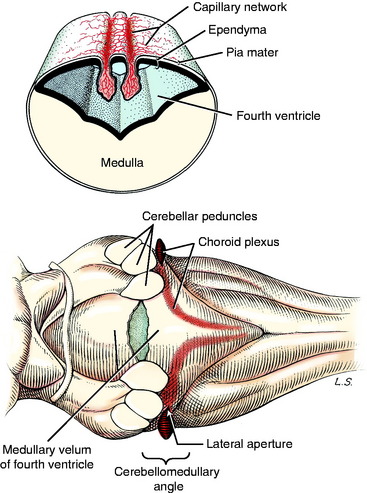
The leptomeninges that surround the entire developing central nervous system (CNS; neural tube) arise from neural crest cells and adjacent mesodermal cells. These meninges contain the bulk of the blood vessels that supply the CNS and the roots of the peripheral nerves. Dorsal to the stretched-out roof plate of the fourth ventricle, the capillary blood vessels proliferate to form the two longitudinal rows of a dense capillary bed. The adjacent ependymal cells enlarge into cuboidal cells, and the entire structure (cuboidal ependymal cells, pia mater, and capillary bed) hangs down into the lumen of the fourth ventricle (Fig. 3-10). This is called the choroid plexus of the fourth ventricle. By strict definition, only the proliferated capillary bed is the plexus. Thus the choroid plexus of the fourth ventricle comprises two sagittal lines parallel to and on either side of the median plane. These extend from the caudal part of the fourth ventricle rostrally to the level of the cerebellar peduncles where each plexus turns laterally. At this point there is an opening that develops in the medullary roof plate, called the lateral aperture. This aperture allows communication between the lumen of the fourth ventricle and the subarachnoid space that develops in the leptomeninges. At the level of this lateral aperture, the choroid plexus protrudes from the lumen of the fourth ventricle out through the aperture, where it is visible on each side at the cerebellomedullary angle (see Fig. 3-10). The aperture itself is invisible grossly because it is filled with this choroid plexus. The choroid plexus is a major site of formation of cerebrospinal fluid (see Chapter 4). In domestic animals, the lateral aperture is the only communication between the ventricular system of the brain and the subarachnoid space, which makes it critical for the maintenance of normal intracranial pressure. Primates have an additional aperture located caudally in the caudal medullary velum of the fourth ventricle.
METENCEPHALON: CEREBELLUM AND PONS (See Figs. 2-9 and 2-10)
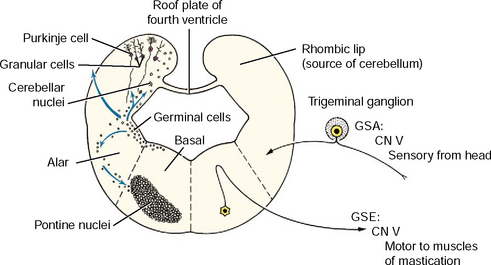
The initial development of the metencephalon is comparable to that of the myelencephalon. Cranial nerve V, the trigeminal nerve, is associated with this segment of the brainstem (Fig. 3-11). Its motor neurons arise in the basal plate mantle layer, migrate a short way ventrolaterally into the parenchyma of the pons, and form a small, well-defined motor nucleus. These neurons function in the general somatic efferent system and innervate the muscles of mastication derived from the somitomeres in branchial arch 1. The sensory neurons in cranial nerve V are derived primarily from neural crest cells and form the trigeminal ganglion. Most of these neurons are GSA and their dendritic zones are widely spread over the entire surface of the head and to the inner surface of the upper respiratory and digestive systems. A smaller component is composed of general proprioceptive neurons for the muscles and joints in the head region. These sensory neurons greatly outnumber the motor neurons. Therefore, when these sensory axons enter the alar plate region of the metencephalon, they spread out for a short distance rostrally and for a long distance caudally, forming the spinal tract of trigeminal nerve in the pons and medulla. These axons terminate in telodendria at synapses in the alar plate neurons, which form the sensory pontine nucleus of the trigeminal nerve in the pons and the nucleus of the spinal tract of the trigeminal nerve in the medulla. This spinal tract and nucleus extends the full length of the medulla, where caudally they meet the comparable functional neurons developing in the first cervical spinal nerves and spinal cord segment (see Fig. 3-11).
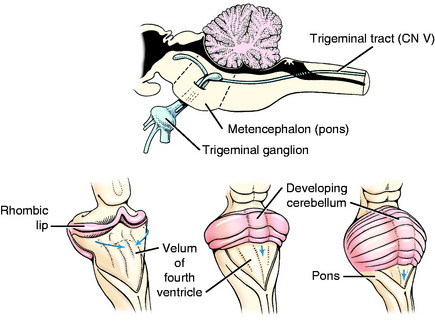
Figure 3-11 Development of the metencephalon: surface view and sagittal view of the afferent portion of cranial nerve V.
The cerebellum, or dorsal metencephalon, is formed primarily from the proliferation of the germinal epithelial cells of the alar plate, forming the rhombic lip (see Figs. 3-11 and 3-12). This growth dorsolaterally from each side overgrows the roof plate of the fourth ventricle so that the cerebellum forms part of the dorsal boundary of the fourth ventricle in the metencephalon. The development of the cerebellar cortex and nuclei are described in Chapter 13. The ventral metencephalon is the pons. A ventral migration of alar plate mantle layer neurons forms the pontine nucleus (see Fig. 3-12). The axons of these neurons cross the midline and course dorsally into the cerebellum. This forms the transverse fibers of the pons, which demarcate the ventral surface of the pons and the middle cerebellar peduncle.
MESENCEPHALON: MIDBRAIN (See Figure 2-5 through 2-9)
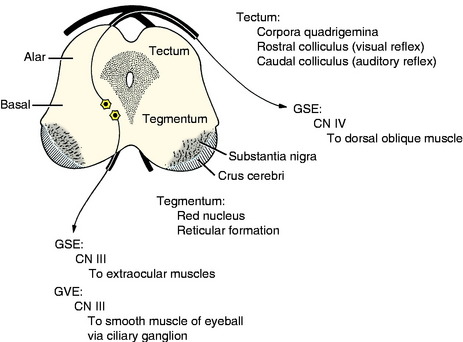
Cranial nerves III (oculomotor) and IV (trochlear) are associated with the midbrain. These contain primarily GSE neurons that innervate extraocular muscles. The cell bodies are in nuclei derived from the basal plate mantle layer. They do not migrate but remain adjacent to the median plane ventral to the aqueduct, which is in the same topographic nuclear column as the abducent and hypoglossal GSE nuclei in the medulla (Fig. 3-13). The oculomotor nucleus also contains the neuronal cell bodies of the preganglionic parasympathetic innervation to the constrictor muscle of the iris. These are derived from the same basal plate mantle layer.
The alar plate proliferates dorsally to form the tectum of the midbrain, which is divided into paired rostral and caudal colliculi, collectively known as the corpora quadrigemina. These are associated with visual and auditory reflex function, respectively. The crus cerebri on the ventral surface of the midbrain results from the caudal growth of descending axons from telencephalic projection neurons. These are continuous from the internal capsule in the diencephalon.
DIENCEPHALON: INTERBRAIN (See Figure 2-3 through 2-6)
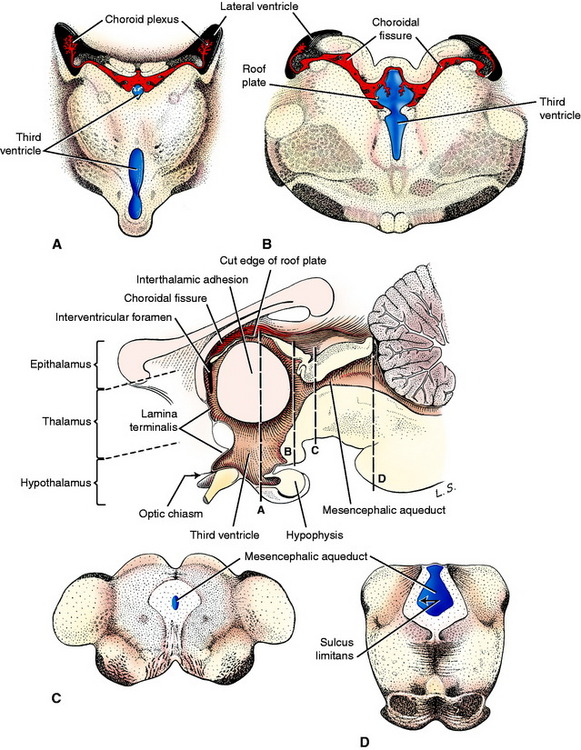
Rostral to the mesencephalon, the sulcus limitans is no longer evident in the neural tube and the diencephalon and telencephalon are considered to be developments of the alar plate. The symmetric development of the lateral walls of the neural tube in the diencephalon reduces the neural canal to a vertical slit on the median plane, the third ventricle. Adhesion of the developing thalamus in the center forms the interthalamic adhesion and separates the third ventricle into a small dorsal component and a larger ventral component. These two portions converge caudally at the mesencephalic aqueduct and rostrally at the level of the interventricular foramina, which connect to each telencephalic lateral ventricle (Fig. 3-14). On the dorsal median plane of the diencephalon, there is no proliferation of the neural tube epithelial cells, leaving a single-cell-thick roof plate, where a small choroid plexus develops in two parallel lines similar to those in the medulla. At the interventricular foramina each of these is continuous with the choroid plexus that develops in each lateral ventricle (Fig. 3-15).
These nuclei form the thalamencephalon, hypothalamus, and subthalamus. The thalamencephalon consists of the thalamus, metathalamus, and epithalamus, which comprise those nuclei located dorsal to the ventral portion of the third ventricle. The hypothalamus includes the nuclei located on the sides and floor of the ventral portion of the third ventricle. The subthalamic nuclei are located ventrolaterally in the diencephalon. A ventral outgrowth of the hypothalamus, including an extension of the third ventricle, forms the neurohypophysis. The neurohypophysis becomes associated with a dorsal extension of the adjacent oral ectoderm, the hypophyseal (Rathke’s) pouch, to form the hypophysis (pituitary gland). The optic vesicles that initially grew out of the prosencephalon will form the neural layer of the eye and optic nerves, which become associated with the diencephalon (see Fig. 3-3). The axons that grow caudally from the retina in the optic nerve will form the optic tracts of the diencephalon, and many will terminate in a nuclear area of the thalamus. These optic nerves form cranial nerve II, the special somatic afferent neurons of the visual system. By definition, a nerve is a collection of axons outside the CNS that are myelinated by Schwann cells, which arise from the neural crest. Optic nerves are misnamed because they develop as extensions of the prosencephalon. They form in the optic stalk that extends from the diencephalon to the optic cup. Their axons are myelinated by CNS oligodendroglial cells. This is important to remember because the optic nerves are affected by diseases that are specific to the CNS. Therefore, optic neuritis is a form of encephalitis. A polyneuritis does not affect the optic nerves.
TELENCEPHALON: CEREBRUM (See Figure 2-2 through 2-7)
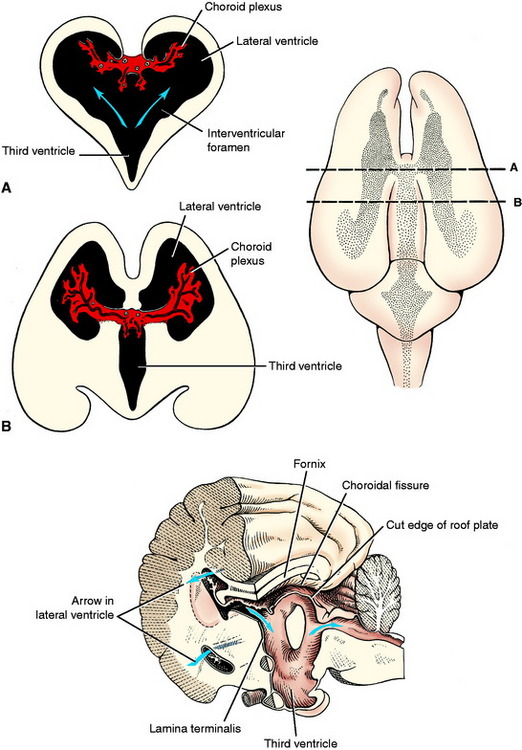
The rostral boundary of the brainstem is the lamina terminalis of the diencephalon. It is the rostral boundary of the third ventricle. The optic chiasm is located at the ventral portion of this lamina, and the rostral commissure develops in and remains in this lamina. It is at this level that the telencephalic vesicles grow out of the original prosencephalon to form the two cerebral hemispheres that comprise the cerebrum. The lamina terminalis is located on the median plane between these two outgrowths. The telencephalic vesicles grow out of the prosencephalon a short distance rostrally and then in a large curve caudally and ventrally. The neural canal in each telencephalon is the lateral ventricle, which communicates with the diencephalic third ventricle via the interventricular foramen on each side of the lamina terminalis (see Figs. 3-15 and 3-18).
At one aspect of the medial wall of the telencephalic vesicle, the neuroepithelial layer of the neural tube does not proliferate and remains a single layer of cells that become ependymal cells comparable to the roof plate of the myelencephalon over the fourth ventricle and the roof plate of the diencephalon over the third ventricle. As the rest of the telencephalon proliferates and differentiates, this telencephalic roof plate will be attached to the crus of the hippocampal fornix on one side and the stria terminalis on the other side. The choroid plexus of each lateral ventricle develops in this roof plate as was described for the choroid plexus in the medulla. This is a curved structure similar to the structures that it is attached to, and it protrudes into the lumen of the lateral ventricle. At each interventricular foramen, each lateral ventricular choroid plexus is continuous with the choroid plexus of the third ventricle.
Telencephalic neuronal cell bodies and white matter can be organized as follows.
Cell Bodies
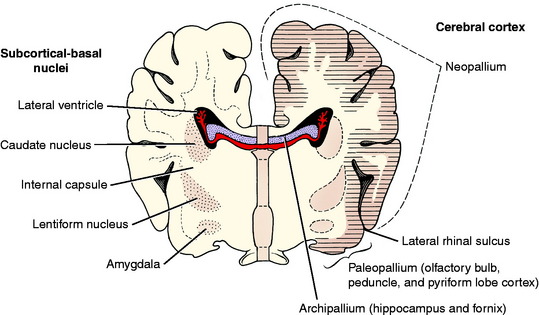
The telencephalic neuronal cell bodies are located in one of two general locations. One is on the external surface of the entire telencephalon, forming the various layers of the cerebral cortex. The other is deep to the surface in subcortical basal nuclei. These are often incorrectly called basal ganglia. Remember that ganglia are collections of neuronal cell bodies outside the CNS. Such collections inside the CNS are nuclei or cortices. Cortices are located superficially, and their neuronal cell bodies are in a continuous arrangement. Examples of basal nuclei are the caudate nucleus, globus pallidus, putamen, claustrum, and amygdaloid body. The cerebral cortex can be divided into three regions based on evolutionary and anatomic features. The archipallium (pallium is a synonym for cortex) is the hippocampus, which is an internal gyrus, an area of cerebral cortex that has been rolled into the lateral ventricle and is not visible on the surface of the cerebrum. The paleopallium is the olfactory system and is composed of the olfactory bulbs, the peduncles, and the piriform lobe cortex. In animal evolution, these are the most primitive portions of the cerebrum. The neopallium is a more recent evolutionary brain development and makes up the surface of all the gyri of the cerebrum (Fig. 3-16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree