(1)
Arezzo, Italy
4.1 Introduction
The primary neoplasms of the skin are discussed in this chapter. Skin metastases originating from internal malignancies are not included in this wide group of neoplasia, as they will be briefly covered in the Chap. 5. With rare exceptions, such as ulcerative feline squamous cell carcinoma and exfoliative erythematous dermatitis in some cases of canine epitheliotropic lymphoma, almost all skin neoplasia are nodules or plaques. For this reason, an approach based on clinical signs, as used for inflammatory skin diseases, is not possible. Therefore, to discuss the cytology of skin neoplasms, a classification based on the cytological and histological morphology of the neoplastic cells must be used.
In the “WHO Histological Classification of Tumours of Domestic Animals” published in 1998, skin tumours are divided into epithelial, melanocytic, mesenchymal and unclassifiable; in addition to these four groups, metastatic tumours to the skin, cysts, hamartomas and neoplastic-like lesions are also included. This classification is obsolete as it does not include many skin tumours, which are now well documented in many articles and books on histopathology and clinical oncology of the skin (Gross et al. 2005; Withrow et al 2012).
In recent decades, the study of histopathology and the increased use of immunohistochemical stains, allowed a broader and more detailed classification of tumours of the skin of pets (Yager and Wilcock 1994; Gross et al. 2005). At the same time, veterinary neoplastic cytology has made great strides, which has led to the publication of many books focused on general and skin cytopathology (Baker and Lumsden 1999; Valenciano and Cowell 2014; Raskin and Meyer 2015). Furthermore, a number of articles supporting the accuracy of cytology in the diagnosis of skin cancer and its high correlation with histopathological findings have been published (Griffiths et al. 1984; Chalita et al. 2001; Cohen et al. 2003; Ghisleni et al. 2006). All these factors have led to the routine use of cytology as a mandatory step in the diagnosis of skin lesions; the results of the cytology may in fact define the nature of a tumour and direct the veterinarian in selecting the right tests to be performed for proper pre-surgical staging of the tumour.
The textbooks on skin cytology and dermatology in dogs and cats use a “hybrid” cytological classification of skin cancers; indeed, although for certain cancers a definition based on the origin of the cells and/or their architectural arrangement (e.g. epithelial and mesenchymal tumours) is used, other tumours are grouped based exclusively on their morphology (e.g. round cell tumours). Tumours of melanocytic origin, such as melanocytoma and melanoma, can increase confusion in this classification, as the neoplastic melanocytes are characterised by a remarkable polymorphism; indeed, they can be round or spindle–shaped, or organised in an epithelioid arrangement. For this reason, they are included in a separate group under the heading melanocytic tumours. This cytological classification does not reflect that used in histopathology (Gross et al. 2005); moreover, a cytological approach focused on the morphology of the cells allows the veterinarians to follow a simplified algorithm aimed at more rapid achievement of the diagnosis. It should be stressed that regarding neoplastic cytology, the cells do not always respect the morphological rules on which the above classification is based, as is exhaustively discussed in this chapter.
In this textbook, the skin neoplasms are divided into four groups:
Round cell tumours
Epithelial tumours
Mesenchymal tumours
Melanocytic tumours
4.2 Round Cell Tumours
Round cell tumours, although originating from different cell lines, share some cytological characteristics: the high number of cells yielded, the roundish silhouette with well-defined cytoplasmic margins and the trait of being discrete, meaning that they do not aggregate with each other or to an extracellular matrix. Although the above characteristics are observed in the majority of cases, some exceptions are recognised. A typical example is the histiocytic sarcoma, which belongs to the round cell tumour group (histiocytic diseases); in the spindle cell subtype of this neoplasia, the cytological features that allow the cells to be defined as round cells are not observed. In contrast, there are other skin cancers such as liposarcomas, melanomas or some poorly cohesive anaplastic carcinomas, which, although they are not included in this group, may present as round cells. From the above, it is deduced that this cytological classification is not free from risks of interpretation and should only be used as a diagnostic orientation, and, if diagnosis has not been achieved through cytology, further histopathological and sometimes immunohistochemical investigations must be performed.
Tumours included in this group are mast cell tumours, lymphomas, plasma cell tumours, transmissible venereal tumours and the so-called histiocytic diseases.
4.2.1 Mast Cell Tumour
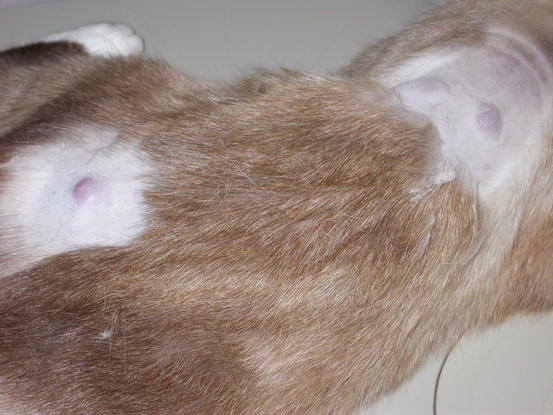
Mast cell tumours (MCTs) are very common in dogs while they are less frequent in cats (Goldschmidt and Hendrick 2002; Welle et al. 2008). MCTs originate from dermal mast cells. In dogs, mast cell tumours are the most common form of skin cancer and can appear as nodules or plaques of varying sizes, ranging from a few millimetres up to large masses. The nodules are usually single, but can be multiple, dermal or subcutaneous in location, dome- or button-shaped, pedunculated, alopecic, erythematous or hyperpigmented, firm, soft or gelatinous in consistency and sometimes ulcerated (Figs. 4.1, 4.2, 4.3, and 4.4). Adults and old dogs are most commonly affected, but rarely, single or multiple MCTs can also be observed in very young animals. Many canine breeds, such as Boxer, Shar-pei, Labrador, Golden retriever, Boston terrier etc., are strongly predisposed; in Shar-pei dogs, which have a high quantity of mucin in the dermis, the MCTs frequently present as swellings with undefined margins (Fig. 4.5) (Blackwood et al. 2012).
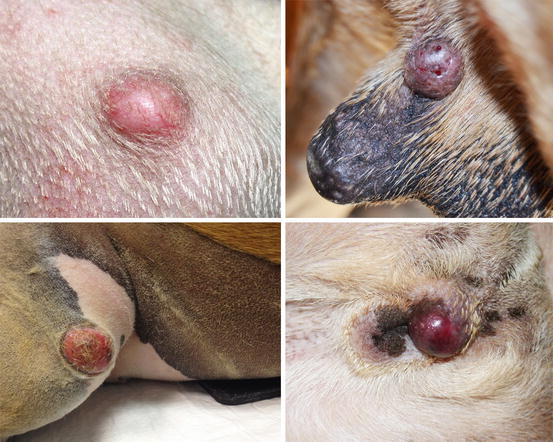
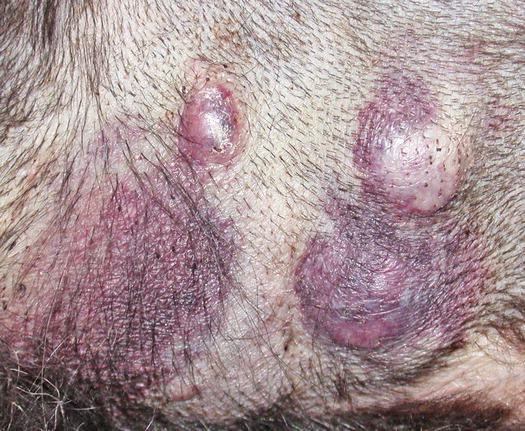
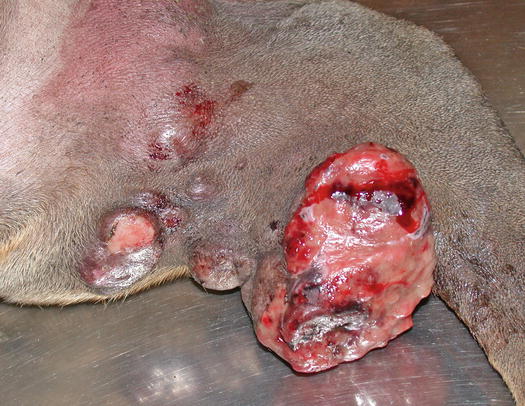
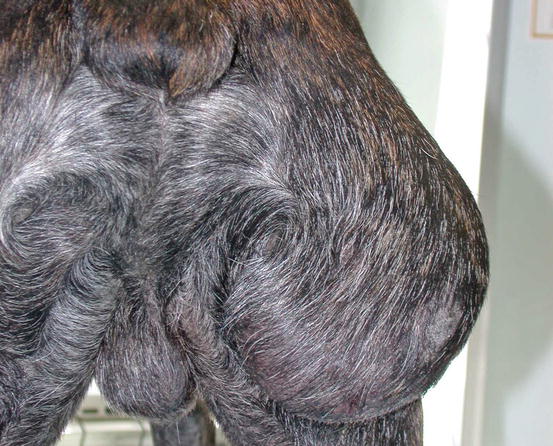
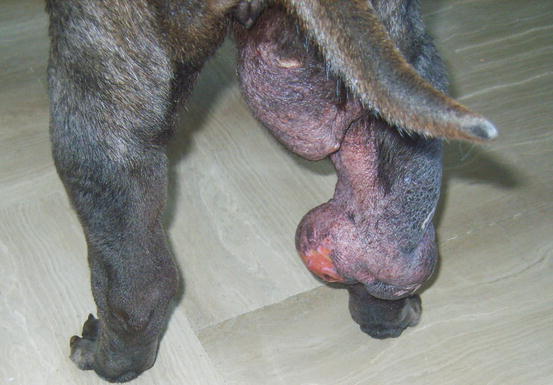

Fig. 4.1
Canine mast cell tumours (MCTs): single alopecic and erythematous nodules

Fig. 4.2
Multiple MCTs on the chest of a dog

Fig. 4.3
Canine MCTs: multiple ulcerated masses on the leg of a Boxer

Fig. 4.4
Large mass, of pasty consistency, on right rump of a Boxer

Fig. 4.5
Diffuse swelling with indistinct margins is a typical clinical presentation of MCTs in Shar-pei dogs
A transitory change in the size occurring after a slight trauma, such as a FNB, is commonly observed in many canine MCTs; this clinical behaviour is unique to MCTs, in which nodules tend to increase in size and then, after a few hours, return to the original size. This phenomenon is due to the degranulation of mast cells, causing the release of intragranular vasoactive substances, which results in an intra- and peri-lesional oedema (Fig. 4.6). In some cases, as result of this degranulation, diffuse erythema of the skin covering the tumour can be observed (Darier’s sign; Fig. 4.7).
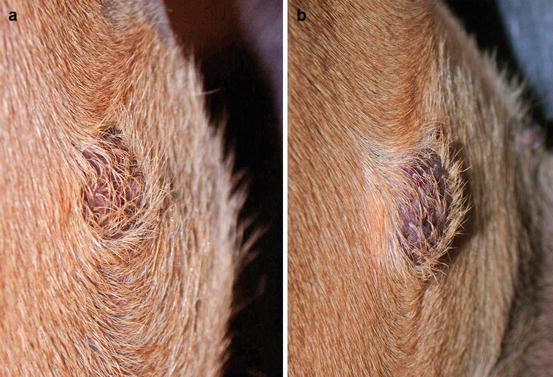
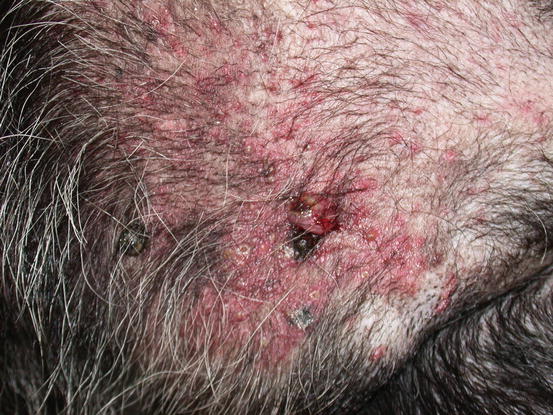

Fig. 4.6
Canine mastocytoma. Size modification during fine needle biopsy (FNB): (a) before and (b) after the sampling. Note how the nodule has increased in size

Fig. 4.7
Redness of the skin around an MCT characterises Darier’s sign
Feline MCT usually occurs as a small, single, alopecic, pinkish or erythematous nodule, mostly located on the head, ears or extremities (Figs. 4.8 and 4.9). In some patients, multiple nodules or plaques with intact or ulcerated skin are present (Figs. 4.10 and 4.11) (Johnson et al. 2002; Blackwood et al. 2012; Henry and Herrera 2013). The single tumours are usually confined to the skin, whereas the multicentric lesions are often secondary to a metastasis from a primitive splenic or intestinal MCT. In any case, the discovery of a cutaneous MCT must always be followed by the search for a possible primary or metastatic visceral lesion. A clinical variant of MCT affecting young Siamese cats, from a few months to 4 years of age, has occasionally been reported. This subtype, called atypical or poorly granulated MCT, is clinically characterised by the spontaneous regression of the nodules (Figs. 4.12 and 4.13).
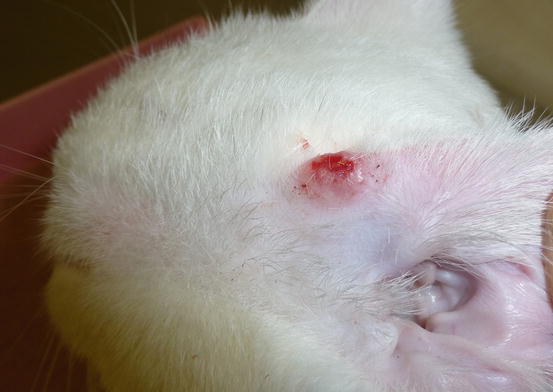
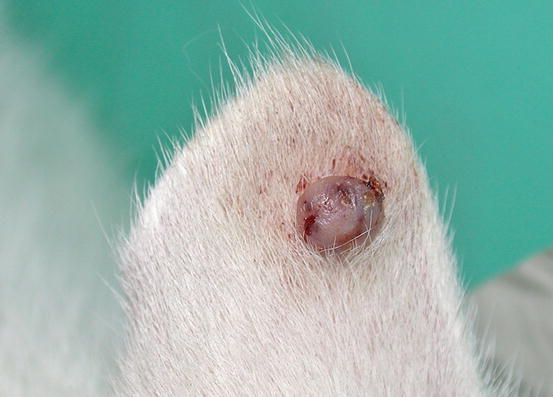
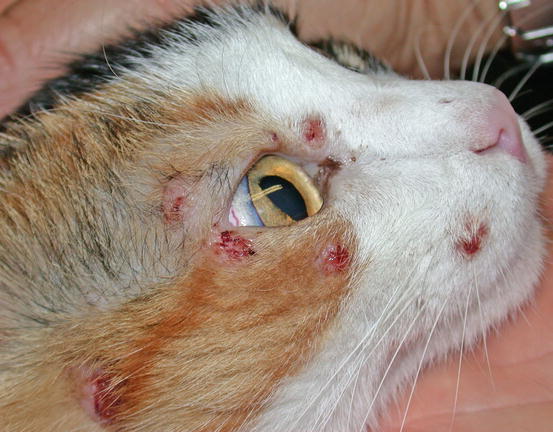
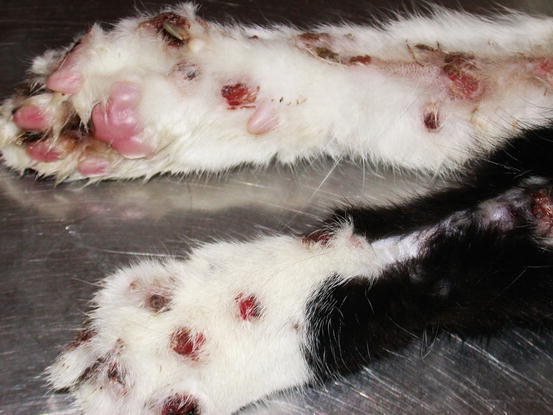
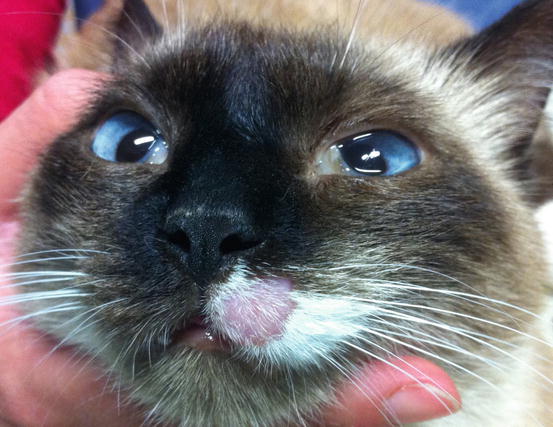

Fig. 4.8
Single and ulcerated MCT at the base of the pinna in a cat

Fig. 4.9
A MCT in a cat: small and alopecic nodule on the pinna

Fig. 4.10
Multiple nodules, some of them ulcerated, on the face of a cat with multicentric MCTs

Fig. 4.11
Multiple metastatic MCTs. The primary neoplasm was located in the spleen

Fig. 4.12
Atypical MCT in a Siamese cat
Cytological Findings
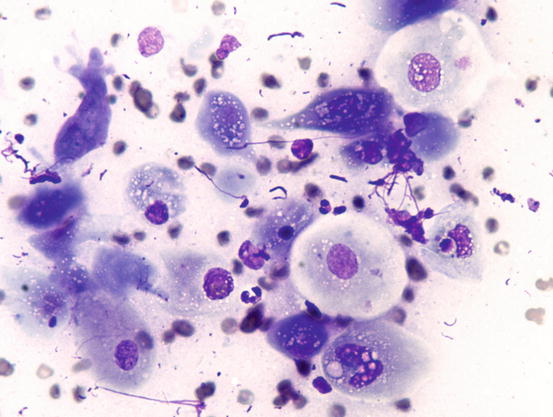
In well-differentiated MCTs, the cytological diagnosis is easy and immediate, especially when proper sampling is performed; slides are usually highly cellular and in 90 % of cases the diagnosis can be made (Fig. 4.14) (Pedraza et al. 2011). In dogs, the MCTs are composed of round cells characterised by many intracytoplasmic granules, which assume a characteristic purple colour with the use of Romanowsky-type staining (Fig. 4.15). In many cells, the granules are so numerous as to completely obscure the nuclei; in other cases, the granules absorb most of the dye and the nuclei are barely noticeable in the centre of the cell as a faded and pale blue round area, surrounded by intensely stained granules (Fig. 4.16). In all the textbooks and papers relating to canine MCTs, the possibility that Romanowsky-type dye is not capable of staining granules is constantly reported. In practice, this phenomenon is a very rare occurrence and, in most cases, a careful observation of the slide allows the detection of intracytoplasmic granules in some cells. In cases where the granules are few or poorly visible, it is possible to highlight them with toluidine blue, which stains the granules red (Fig. 4.17).
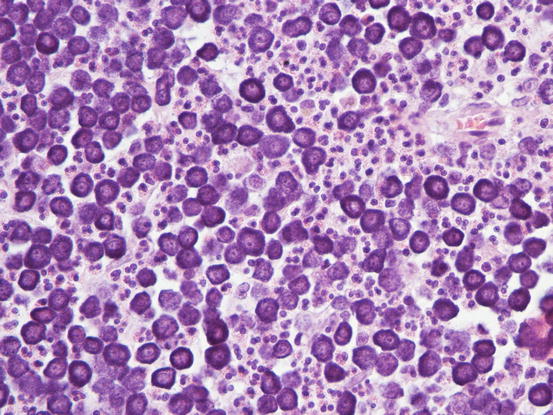
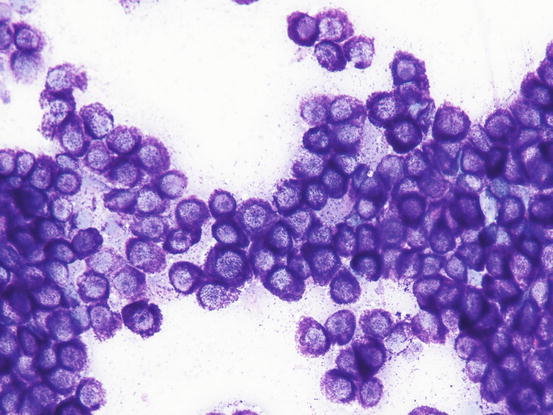
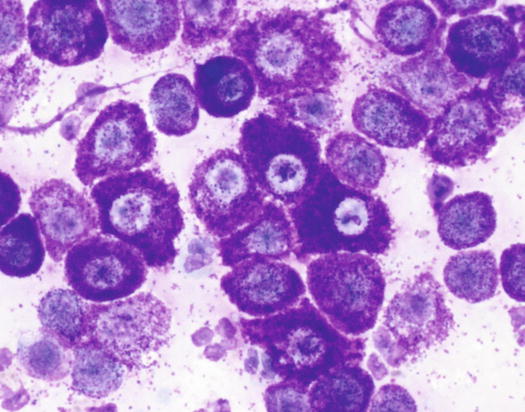
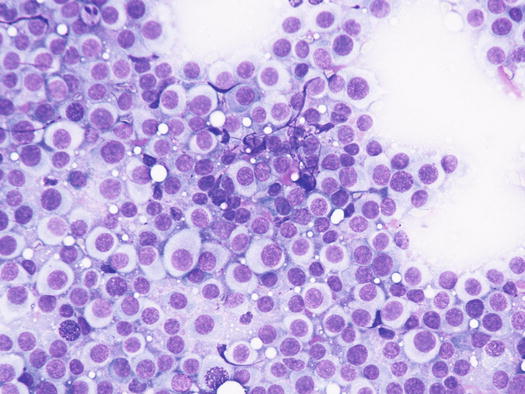

Fig. 4.14
Histopathology of a canine MCT: proliferation of well-differentiated neoplastic mast cells

Fig. 4.15
Cytology of a canine MCT: many well-differentiated mast cells

Fig. 4.16
Cytology of a canine MCT: note the pale, barely evident central nuclei and the intensely purple intracytoplasmic granules

Fig. 4.17
Cytology of a canine MCT: Romanowsky staining. The characteristic purple intracytoplasmic granules have not taken the colour
In many tumours a variable number of eosinophils, from few to many, attracted by the eosinophilotactic substances contained in the granules, are usually observed; eosinophils are therefore of great help in suspecting undifferentiated or poorly granular mast cell tumours. In some tumours, the number of eosinophils is higher compared with the neoplastic cells. In this case, before formulating a diagnosis of eosinophilic inflammation, a histopathology examination of the lesion is mandatory. The number of eosinophils seems lower in anaplastic MCTs (Fig. 4.18).
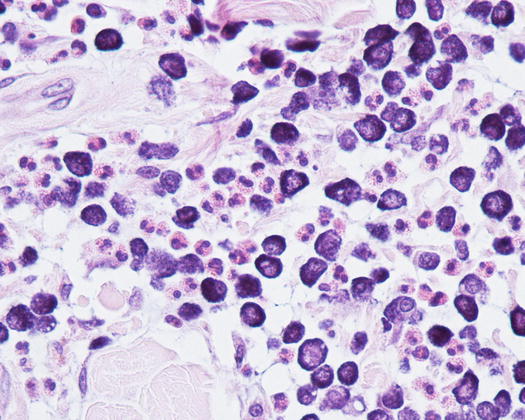

Fig. 4.18
Histopathology of a well-differentiated canine MCT: many eosinophils infiltrate the neoplastic cells
Many reactive fibroblasts are also frequently detected, probably as a result of the release of intracytoplasmic substances that stimulate fibrosis, especially in well-differentiated canine MCTs (Fig. 4.19). Fibroblasts can exhibit cytological abnormalities such as anisokaryosis, double nuclei and prominent nucleoli, and in some samples they can be so numerous that, especially for an inexperienced cytologist, a misdiagnosis of mesenchymal neoplasm could be made. In well-differentiated MCTs, some eosinophilic collagen fibres, probably because of the increase in fibroblasts or following the collagenolytic action of certain substances contained in the cytoplasmic granules, are frequently observed (Figs. 4.20 and 4.21). In so-called keloidal mast cell tumours, the collagen fibres are numerous, thicker and pale pink in colour (Fig. 4.22).
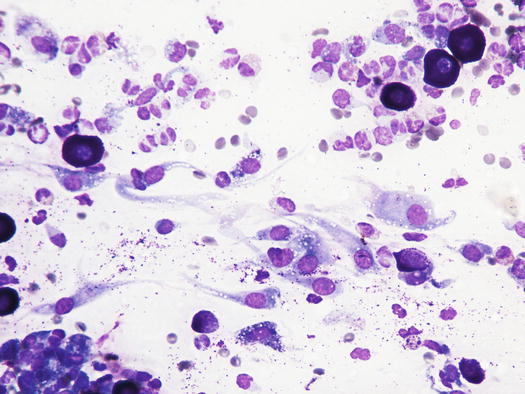
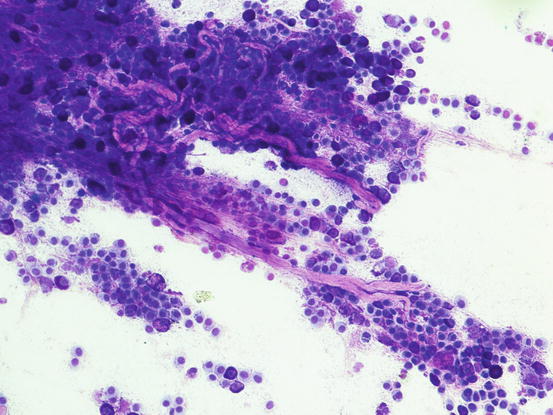
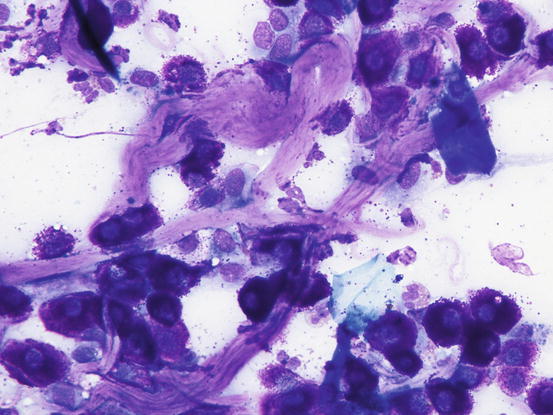
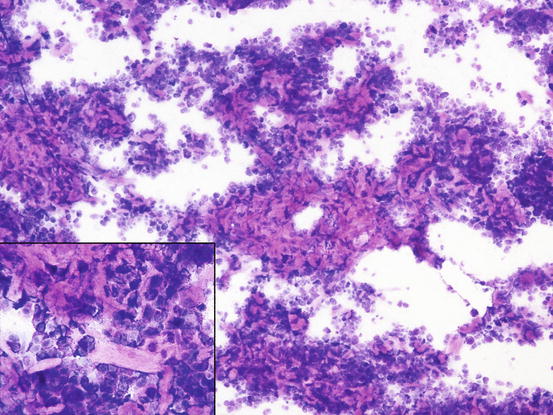

Fig. 4.19
Cytology of a canine MCT: many reactive fibroblasts together with few mast cells and eosinophils

Fig. 4.20
Cytology of a canine MCT: the eosinophilic elongated formations represent collagen fibres. These fibres are very commonly observed in well-differentiated MCTs

Fig. 4.21
Cytology of a canine MCT: at high magnification, the morphology of the collagen fibres is easily recognisable

Fig. 4.22
Cytology of a keloidal canine MCT: many collagen fibres are intermingled within neoplastic mast cells. At high magnification, large and pale collagen fibres are easily recognisable (inset)
In poorly differentiated MCTs, malignant aspects of cells are more evident: the nuclei show more atypia, such as anisokaryosis, can be multiple, with a bizarre shape and with prominent nucleoli (Fig. 4.23). In some cases, the granules are few or completely absent, and cytoplasm can contain multiple micro-vacuoles. The number of atypical mitoses increases. Sometimes in very anaplastic tumours the presence of eosinophils allows the cytologist to suspect an MCT (Figs. 4.24 and 4.25).
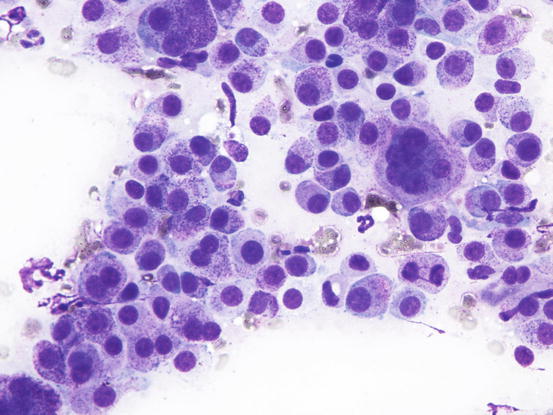
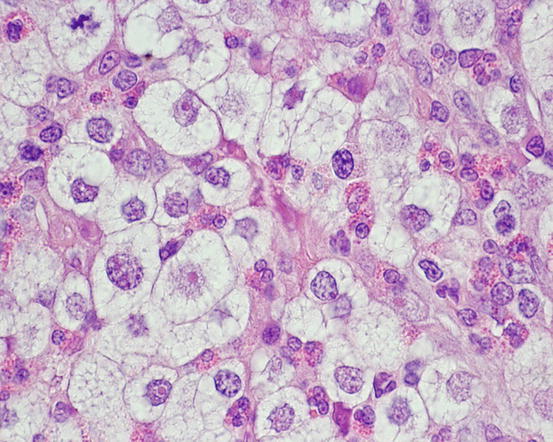
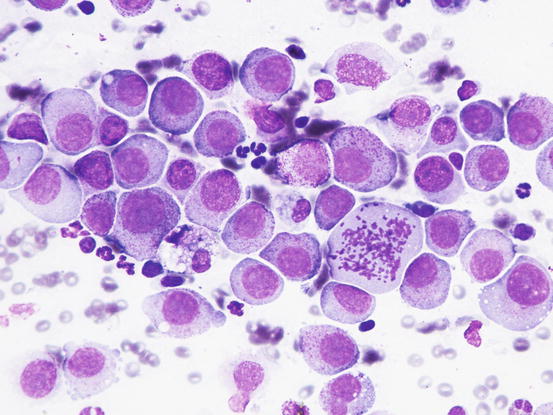

Fig. 4.23
Cytology of a poorly differentiated canine MCT: note the evident features of atypia, such as anisokaryosis, multiple nuclei and atypical mitosis

Fig. 4.24
Histopathology of a poorly differentiated canine MCT: severe cytological atypia. The high number of eosinophils aroused the suspicion of an MCT, which was confirmed by immunohistochemistry

Fig. 4.25
Cytology of a poorly differentiated canine MCT: severe cytological atypia. Scattered purple granules are still detectable in the cytoplasm of some neoplastic cells
Although a cytological evaluation of cellular morphology may provide some indications regarding the degree of differentiation of the MCTs, the definition of the grading remains of exclusive pertinence to the histopathology (Patnaik et al. 1984; Kiupel et al. 2011). Cytologically, when slides show remarkable features of malignancy, a high-grade MCT can be diagnosed, whereas in cases in which MCTs are composed exclusively of well-differentiated mast cells, a low grade can only be hypothesised and histopathological confirmation is mandatory.
The Patnaik histopathological system classifies MCTs into three grades, from I to III. The first two, more frequent grades, in which the cells have cytological aspects indicative of good (grade I) or moderate differentiation (grade II), up to grade III in which the cytological features of atypia increase. In addition to the morphological aspects, this classification is also based on the spread of the neoplastic cells, and for this reason, it is clear how it is not cytologically possible to differentiate between the first two grades of MCT.
In an interesting recent study, the possibility of making a cytological grading of MCTs based on quantitative morphological criteria (number of mitoses, bizarre nuclei, multiple karyomegalic nuclei), which form the basis of the Kiupel’s histopathological classification, has been assessed.
The system attributed to Kiupel and co-authors classifies MCTs as low-grade and high-grade (Kiupel et al. 2011). In this cytological study, the accuracy of predicting the histological grade was 94 %, but it was found to be less reliable in predicting high-grade MCTs. The number of mitoses and the presence of bizarre nuclei are in fact two parameters that offer a high probability of an incorrect cytological evaluation, as the granules often do not allow correct visualisation of the nuclear silhouette. Furthermore, cells can be broken during an improper slide preparation, making it impossible to make a correct assessment of the nuclear shape (bizarre nuclei) and karyomegaly, all parameters included in Kiupel’s classification (Scarpa et al. 2014).
Furthermore, mitotic and multinucleated cells do not have a uniform distribution in the context of neoplastic proliferation and therefore the risk of not being sampled via FNB is very high, rendering the cytological grading less sensitive. Finally, as any MCTs must always be surgically removed and subjected to histopathological investigation, any attempt at grading MCTs through cytology has remained, until now, an unvalidated method. For this reason, cytology cannot substitute for histopathology, particularly because it may underestimate high-grade MCTs because of the very low cut-off of the parameters set by Kiupel’s classification (at least 7 mitosis per 10 hpf; at least 3 multinucleated cells [with 3 or more nuclei] per 10 hpf; at least 3 bizarre nuclei per 10 hpf and karyomegaly) (Kiupel et al. 2011; Scarpa et al. 2014).
Feline MCTs are usually well differentiated and similar to those of the dog. As for the dog, the morphology of feline mast cells does not allow a histological correlation; moreover, in cats, a true grading of MCTs has not yet been validated. There are currently three recognised histological types: mastocytic well-differentiated MCT, mastocytic pleomorphic MCT and the so-called atypical MCT, the latter formerly known by the term histiocytic MCT (Thamm and Vail 2007; Blackwood et al. 2012). To avoid confusion with the nomenclature of feline histiocytic disorders, some authors have therefore suggested abolishing the definition of histiocytic MCT and renaming this atypical subtype using the term poorly granular mast cell tumour (Melville et al. 2014).
The most common subtype in cats is the well-differentiated mastocytic subtype, usually clinically represented by a single nodule. Cells are round to polygonal in shape and usually adhere strongly to each other, giving a pavement-like appearance; the nuclei are usually central and granules are finer and less intensely stained, compared with those of dogs (Figs. 4.26 and 4.27). A variant of this form, considered a subgroup of the well-differentiated mastocytic subtype, is characterised by many multinucleated mast cells, in which the nuclei show a uniform size and the cytoplasm is heavily granulated (Figs. 4.28 and 4.29) (Melville et al. 2014). In poorly differentiated MCTs, the atypical aspects of cells increase and in some cases, a few mature lymphocytes dispersed onto slides can be observed (Gross et al. 2005; Melville et al. 2014; Sabattini and Bettini 2010). In the cat, the atypical or poorly granular subtypes are characterised by cells that morphologically resemble histiocytes, with round or oval large nuclei, sometimes slightly indented, and a larger cytoplasm that contains very few or no granules, and sometimes micro-vacuoles. In rare cases, toluidine blue can stain the rare granules. Some cells can have a spindle shape, which can cause them to be confused with a mesenchymal neoplasm or histiocytic disease. The number of eosinophils, usually scarce in most well-differentiated feline tumours, is often higher in the atypical subtype, and this could be very useful in suspecting a poorly granular MCT (Fig. 4.30).
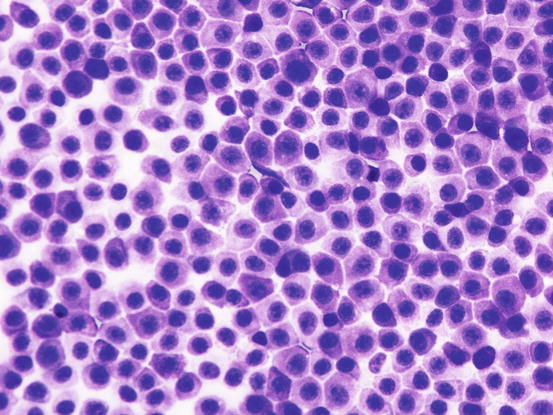
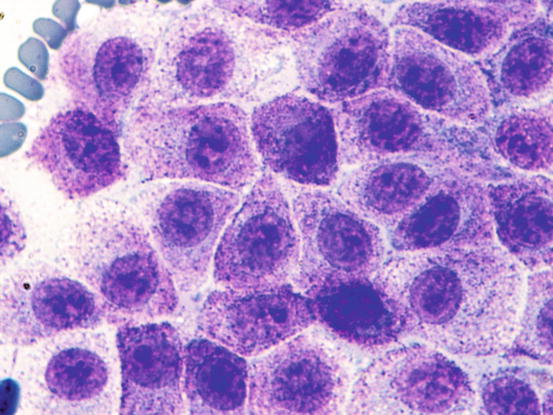
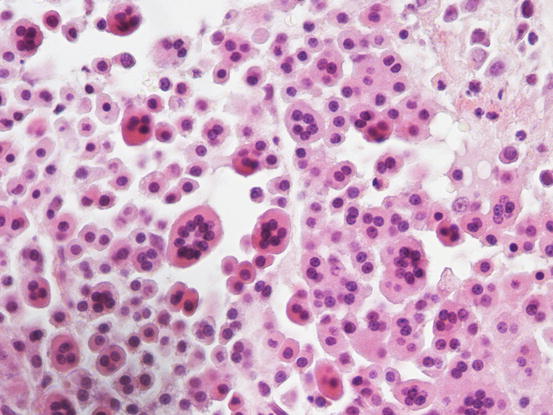
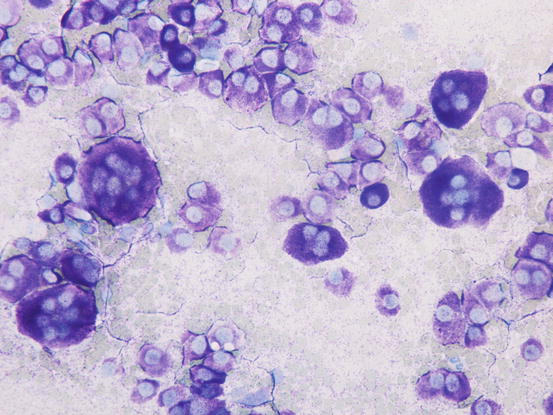
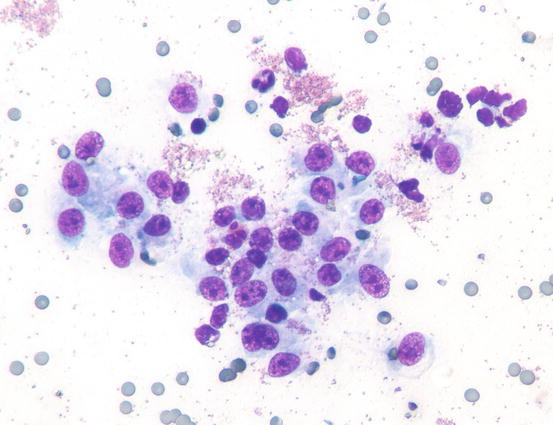

Fig. 4.26
Cytology of a feline mastocytic MCT: highly cellular sample. Note the polygonal silhouette of the feline mast cells

Fig. 4.27
Cytology of a feline mastocytic MCT: at high magnification, the fine purple intracytoplasmic granules are more evident

Fig. 4.28
Histopathology of a feline multinucleated mastocytic MCT: note the high number of multinucleated mast cells

Fig. 4.29
Cytology of a feline multinucleated mastocytic MCT: many multinucleated mast cells

Fig. 4.30
Cytology of an atypical feline MCT: many single cells oval to spindle in shape, with slightly indented nuclei and a granular cytoplasm. Note the high number of eosinophilic granules in the background
4.2.2 Lymphoma
Cutaneous lymphomas are uncommon tumours in dogs and very rare in cats. Histologically, the cutaneous lymphomas are classified as either epitheliotropic, in which the neoplastic cells have a tropism for the epidermis, both external and follicular, or non-epitheliotropic, in which the neoplastic cells are localised in the dermis and can spread to the panniculus (Gross et al. 2005). The phenotype of epitheliotropic lymphoma is always T, whereas the phenotype can be both T and B in the non-epitheliotropic variant. In a recent case report, co-expression of T and B neoplastic cells in a dog with epitheliotropic lymphoma has been demonstrated (Brachelente et al. 2015).
In dogs, the macroscopic aspects of cutaneous lymphoma differ greatly depending on the location of the neoplastic cells.
Epitheliotropic lymphoma is a cancer of adult and old dogs in which different clinical presentations are recognised:
- (a)
Diffuse erythema and desquamation (exfoliative erythroderma),
- (b)
Plaques and nodules,
- (c)
Depigmentation, erythema, erosions and ulcers of the mucocutaneous junctions and nose,
- (d)
Erosions and ulcers localised exclusively to the oral cavity.
Such lesions may occur individually or simultaneously on the same animal (Figs. 4.31, 4.32, 4.33, and 4.34) (Moore et al. 2009: Affolter et al. 2009; Fontaine et al. 2009, 2010; Keller and Moore 2012; Miller et al. 2013).
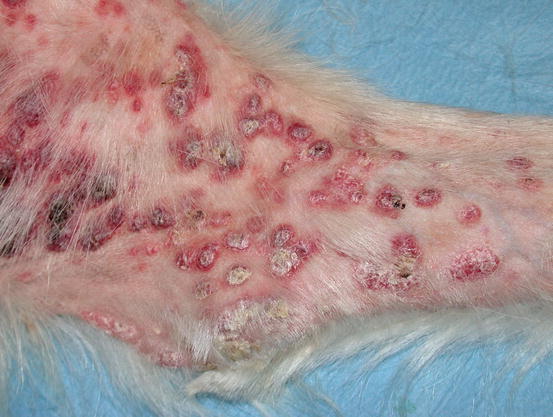
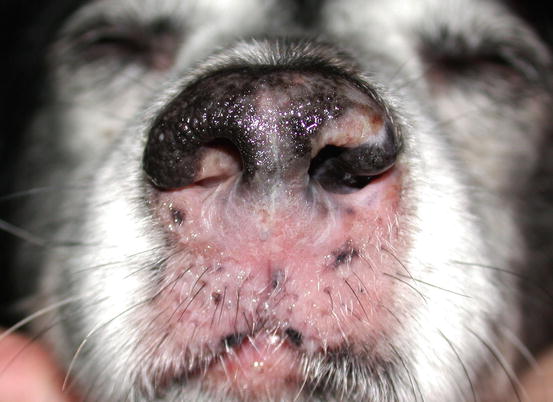
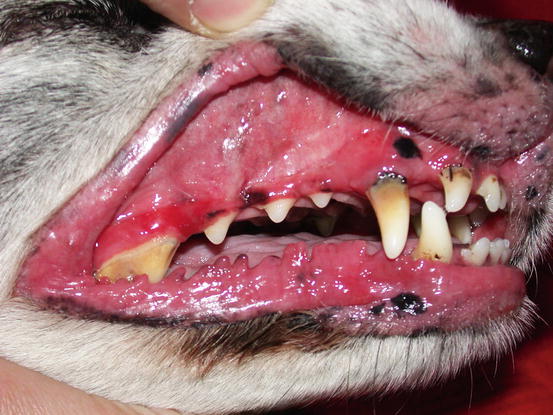

Fig. 4.31
Erythema with large scales in a dog with epitheliotropic lymphoma

Fig. 4.32
Nodular canine epitheliotropic lymphoma: erythematous plaques and nodules on the axilla

Fig. 4.33
Canine epitheliotropic lymphoma: inflammatory depigmentation of the nasal planum and lips

Fig. 4.34
Severe erythema and swelling of the oral mucosa and mucocutaneous junction in a dog affected by canine epitheliotropic lymphoma
In non-epitheliotropic lymphoma, lesions are characterized by multiple, dermal or subcutaneous in location, of different sizes, alopecic, erythematous and often ulcerated nodules and/or plaques (Moore et al. 2013). The more superficial nodules can sometimes assume a bizarre appearance characterised by a donut, arc or serpiginous shape (Figs. 4.35, 4.36, 4.37, and 4.38). This clinical presentation is highly suspicious, but not pathognomonic, for a non-epitheliotropic lymphoma, as it can also be observed in the course of other proliferative disorders such as cutaneous or systemic reactive histiocytosis.
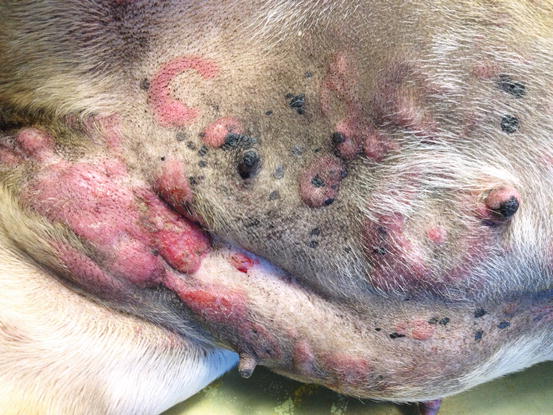
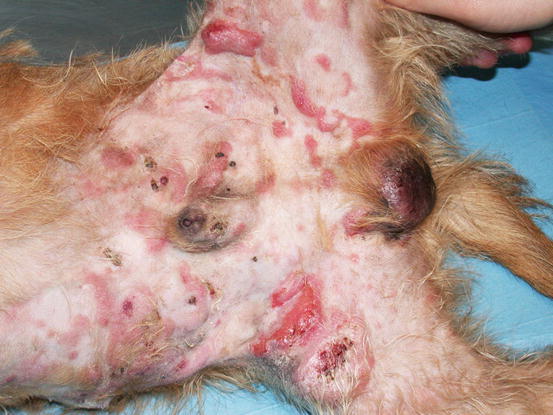
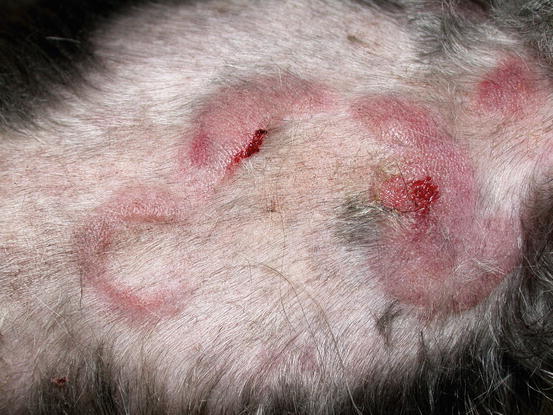
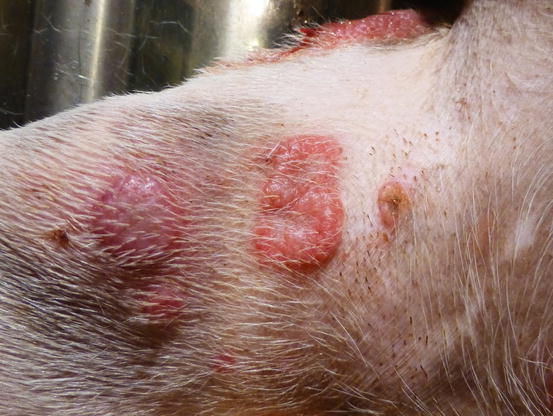

Fig. 4.35
Canine non-epitheliotropic lymphoma: nodules and serpiginous plaques. Note the C-shaped plaque

Fig. 4.36
Canine non-epitheliotropic lymphoma: nodules and serpiginous plaques on the abdomen and legs

Fig. 4.37
Canine non-epitheliotropic lymphoma: at high magnification, the characteristic clinical lesions are more evident

Fig. 4.38
Canine non-epitheliotropic lymphoma: bizarre S-shaped plaque
Feline epitheliotropic lymphoma is extremely rare and is usually grossly characterised by multifocal to generalised alopecia, erythema and desquamation, or by multiple, often ulcerated nodules and plaques of varying sizes; the latter are also typical in non-epitheliotropic lymphoma.
Cytological Findings
As occurs in other round cell tumours, apart from, those cases in which the epitheliotropic lymphoma does not occur as nodules (exfoliative erythroderma, depigmentation of the mucocutaneous junctions), cytopathological samples are usually rich in cells.
In the case of epitheliotropic lymphoma, the quantity of cells collected is strictly linked to the typology of the lesions and the technique of collection performed. The specimens containing the most cells are those sampled from nodular lesions, whereas the exfoliative lesions usually yield specimens with low numbers of cells. In the latter clinical form, cells can only be sampled by imprinting ulcerative lesions. Slides usually have low numbers of cells and, some inflammatory cells, mainly neutrophils, are often associated with the neoplastic cells. In these cases, because in many epitheliotropic lymphomas the cells do not show marked atypia, the coexistence with neutrophils can create mistaken interpretation (Figs. 4.39 and 4.40)
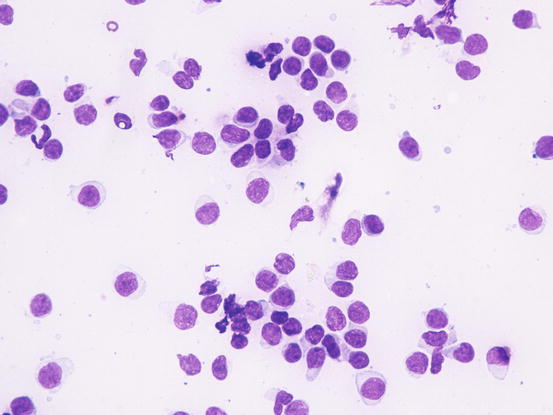
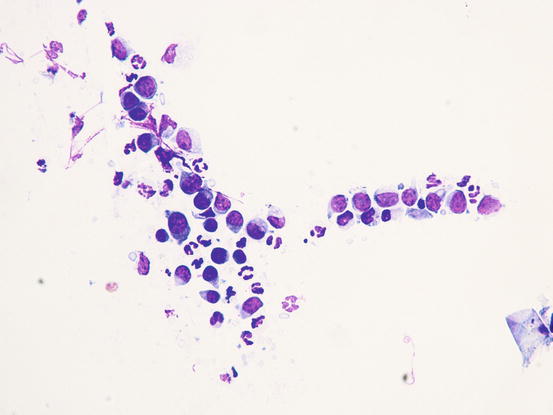

Fig. 4.39
Cytology of epitheliotropic lymphoma: neoplastic lymphocytes of uniform size and with only scarce atypia

Fig. 4.40
Cytology of epitheliotropic lymphoma: neoplastic lymphoid cells associated with neutrophils. Note that the neoplastic cells do not show evident atypia
In the case of nodular lesions, slides are characterised by a homogeneous population of small to medium-sized roundish cells, characterised by round, often slightly indented and sometimes convoluted nuclei with an irregular profile. The nuclear size is fairly uniform, with stippled chromatin, high nucleus/cytoplasm (N/C) ratio, and cytoplasm that shows varying shades of blue, from deep to pale. In non-epitheliotropic lymphomas, cells are usually larger and more pleomorphic (Fig. 4.41).
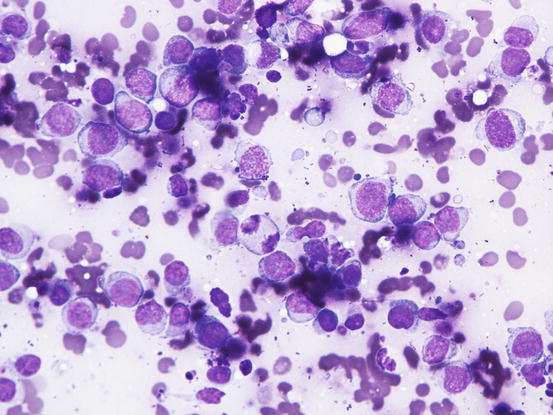

Fig. 4.41
Cytology of non-epitheliotropic lymphoma: large neoplastic lymphoblasts show many features of atypia
In some epitheliotropic lymphomas, cells can be less well-differentiated and anisokaryosis, voluminous nucleoli, multiple and bizarre chromatin patterns are more obvious. As most lymphomas originate from T lymphocytes, it is sometimes possible to observe nuclei with multiple and deep indentations that give the nuclei a cerebriform or petal-like appearance; cytoplasms are larger and paler and may contain basophilic granules (Fig. 4.42). These cytological characteristics can only suggest the T origin of neoplastic lymphocytes, but do not allow the differentiation between epitheliotropic and non-epitheliotropic lymphoma, for which a histopathological examination is mandatory.
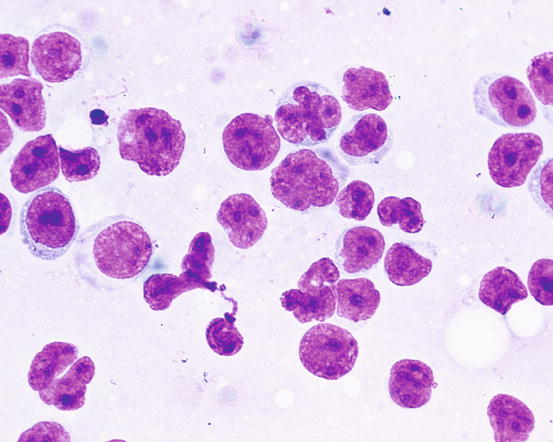

Fig. 4.42
Cytology of epitheliotropic lymphoma: the cerebriform or petal appearance of nuclei suggests their T origin
As above mentioned, when lesions consist exclusively of exfoliative erythroderma, often associated with itching, lesions may be confused with other infectious or allergic diseases (dermatophytosis, leishmaniasis, atopic dermatitis). In these cases, it is possible to try to sample neoplastic cells using intradermal FNAB. This method, described in Chap. 2, does not always allow the collection of diagnostic cells, especially in the case of the pagetoid variant of epitheliotropic lymphoma, in which the neoplastic cells are exclusively located inside the epidermal and follicular wall. Conversely, in the tumoral stages of the disease, when cells diffusely infiltrate the superficial and medium dermis, a variable number of neoplastic cells can be collected via FNAB (Figs. 4.43 and 4.44).
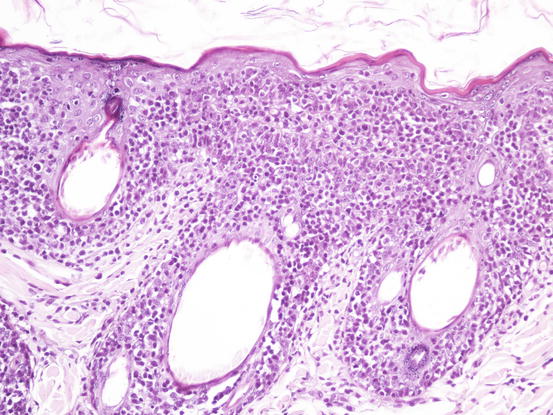
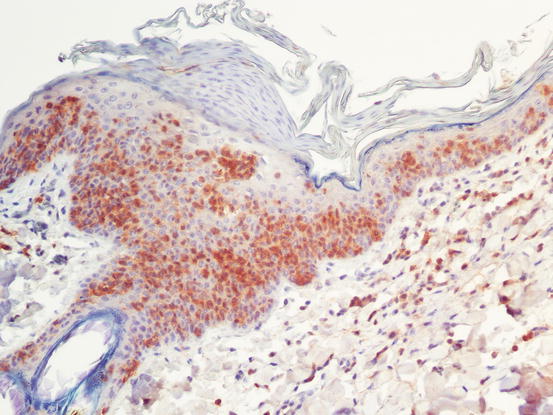

Fig. 4.43
Histopathology of epitheliotropic lymphoma: the neoplastic cells diffusely infiltrate both the external and follicular epidermis

Fig. 4.44
CD3 immunostaining: strong positivity of intra-epidermal neoplastic cells
In the case of non-epitheliotropic lymphomas, histology is characterised by the presence of a diffuse neoplastic lymphoid infiltrating the dermis and the panniculus. When cells are mostly localised in the subcutis, many lipid droplets can be collected, together with a variable number of lymphoblasts. The latters often consist mostly of bare nuclei (without the cytoplasm) and only a small number of cells have the cytoplasm still intact. In these cases, it must be careful to not make a misdiagnosis of panniculitis. The usually high number of lympho-glandular bodies spread on the background helps to interpret the bare nuclei as being of lymphoid origin.
In the presence of a single skin nodule composed of lymphoid cells with neoplastic appearance, care must be taken before making a cytological diagnosis of lymphoma, because spontaneous regression of these lesions can occur (Fig. 4.45).
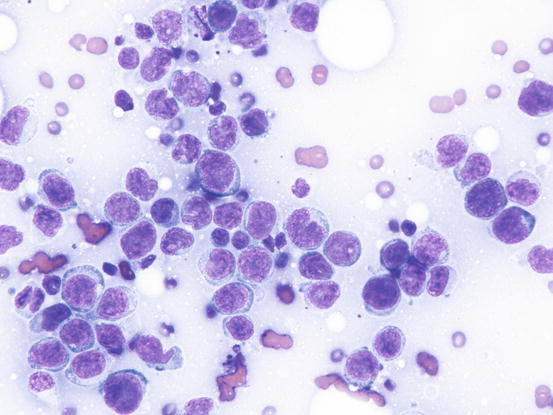

Fig. 4.45
Cytology of non-neoplastic lymphoid cells: note the high number of blasts and the scattered small lymphocytes
The clinical behaviour has allowed such lesions to be defined as non-neoplastic lymphoid inflammatory reactions of unknown cause that could be considered pseudo-lymphomatous lesions. From the above, based exclusively on cell morphology, many single nodules diagnosed as lymphoma only using histopathology could have been undefined non neoplastic lymphomatous proliferation. In these cases, to obtain the definitive diagnosis of lymphoma, the clonality of the cells, which should be monoclonal in neoplasia and polyclonal in reactive lesions, must be assessed. Therefore, the diagnosis of cutaneous lymphoma using cytology should always be made with caution, especially when only a single nodule is present.
4.2.3 Plasma Cells Tumour
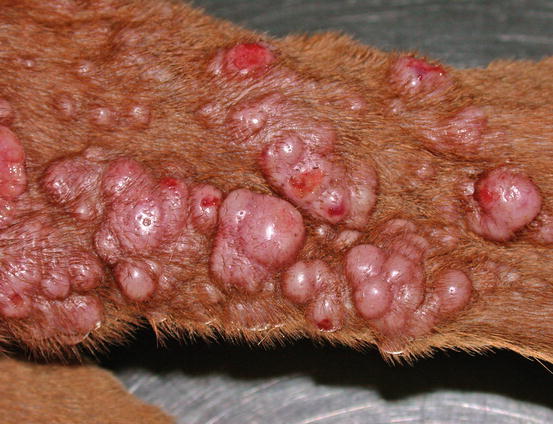
Extra-medullary or cutaneous plasma cell tumour or plasmacytoma is an uncommon neoplasia in dogs and rare in cats. Canine cutaneous plasmacytoma usually develops as single and more rarely multiple alopecic nodules that are often pink-reddish in colour. Lesions are of varying sizes and shapes, located mainly on the toes, pads, pinna, nose, lips and in the mouth (Figs. 4.46, 4.47, 4.48, and 4.49). In cats, they are very rare tumours and are characterised by a single nodule, mostly reported on the legs, face and tail.
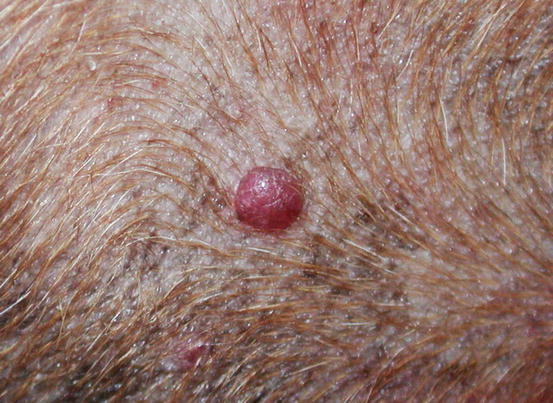
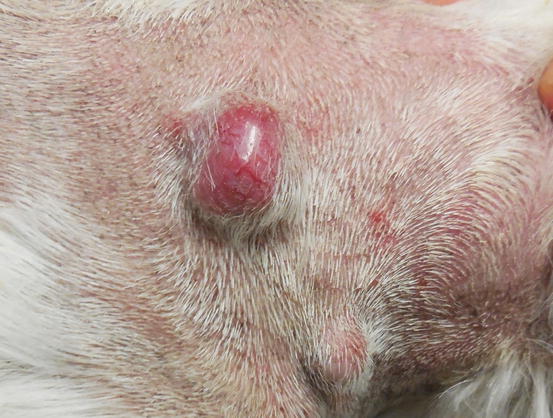
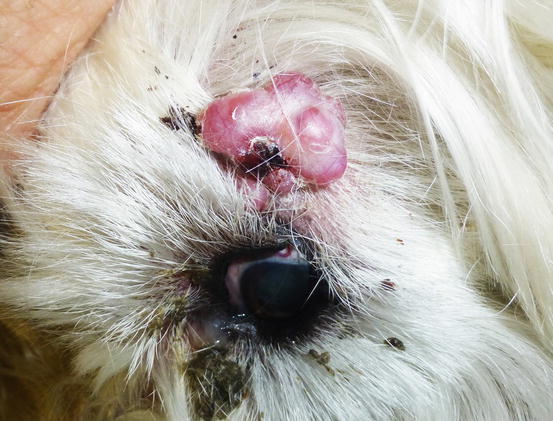
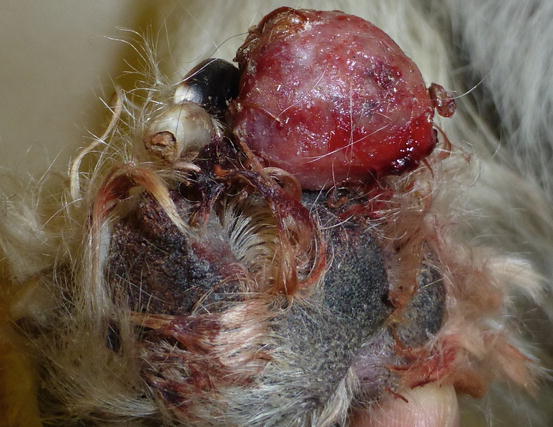

Fig. 4.46
Small erythematous cutaneous plasma cell tumours

Fig. 4.47
Two cutaneous plasma cell tumours

Fig. 4.48
Extramedullary plasma cell tumours with an irregular shape on the eyelid of a West Highland white terrier

Fig. 4.49
Plasma cell tumours: large ulcerated and bleeding tumour on a digit
Cytological Findings
Canine cutaneous plasmacytoma is classified into five histological subtypes and therefore, the polymorphism of this tumour is remarkable. The five histotypes are: hyaline, mature, cleaved, asynchronous and polymorphous–blastic (Figs. 4.50 and 4.51) (Platz et al. 1999; Cangul et al. 2002; Gross et al. 2005). This detailed classification based on the cytological morphology does not seem to have any strong implications regarding the biological behaviour of the tumour. In cats, there appears to be four subtypes (Majzoub et al. 2003). Cytologically it is not always possible to recognise to which histotype the cells belong, also because in some cases, different histological types can coexist in the same tumour. Therefore, from a cytological point of view it is only possible to classify the grade of differentiation based on cell morphology, and define whether the neoplasia is well- or poorly differentiated or pleomorphic; the former are characterised by a monomorphous population of small cells, with roundish or oval eccentric or central nuclei, regular and sometimes stippled chromatin and, in some cells, an achromatic area between the nucleus and the cytoplasm is evident. This area represents the Golgi complex (Figs. 4.52 and 4.53). Some binucleated and a few multinucleated cells are a normal finding in well-differentiated plasma cell tumours (Fig. 4.54). These cytological features that permit the suspicion of a plasmacellular origin, are usually not evident in pleomorphic plasmacytomas, in which cells show varying degrees of anisocytosis, anisokaryosis, kidney-shaped or bizarre nuclei and a large number of multinucleated cells (Figs. 4.55 and 4.56). In these tumours, a small percentage of lymphocytes and eosinophils can be observed.
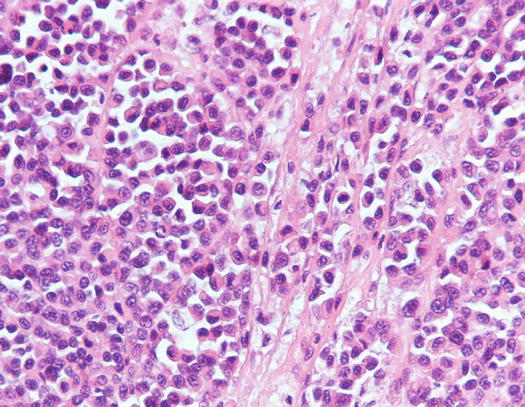
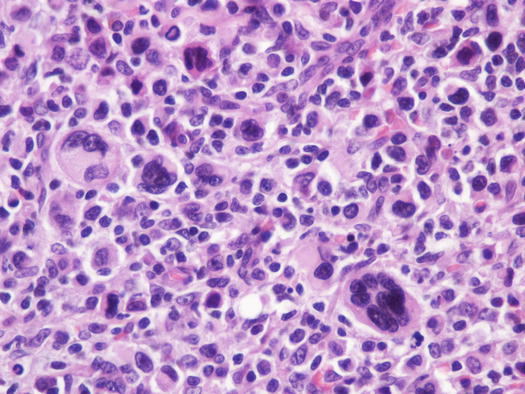
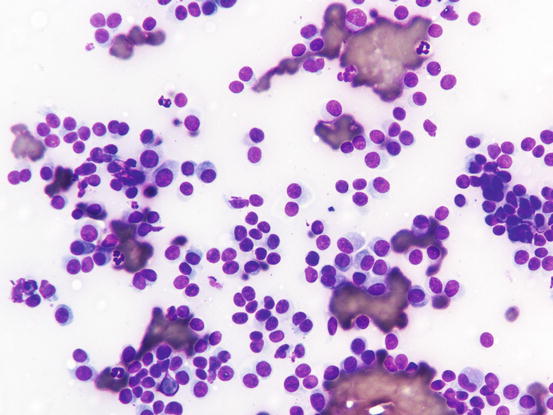
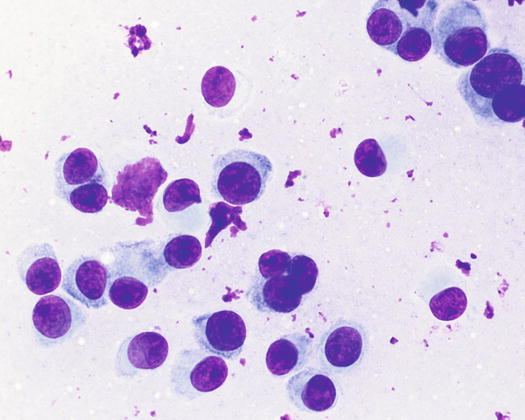
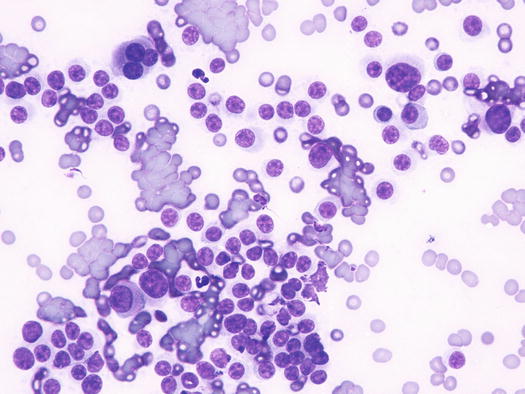
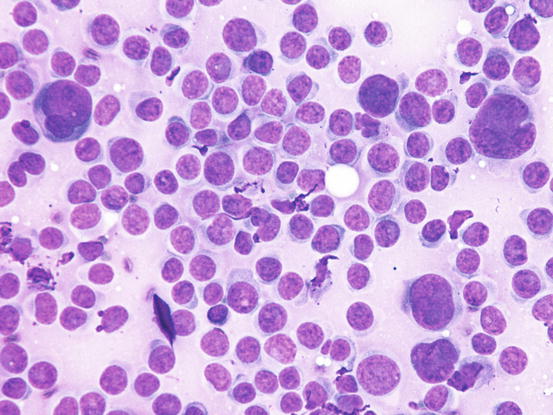
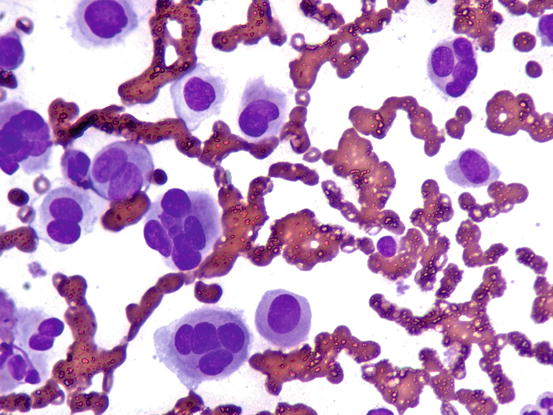

Fig. 4.50
Histopathology of well-differentiated plasmacytoma: small and uniform-sized neoplastic plasma cells, in which the silhouette resembles mature plasma cells

Fig. 4.51
Histopathology of pleomorphic plasma cell tumour: round and discrete neoplastic plasma cells with severe atypia

Fig. 4.52
Cytology of well-differentiated cutaneous plasma cell tumours: small, uniform-sized neoplastic plasma cells. Note that some cells preserve a recognisable plasma cellular morphology

Fig. 4.53
Cytology of well-differentiated cutaneous plasma cell tumours: at high magnifications the plasma cellular morphology is more evident. Note that in some cytoplasm, the Golgi complex is still recognisable

Fig. 4.54
Cytology of poorly differentiated cutaneous plasma cell tumours: anisocytosis, anisokaryosis and multinucleated cells. Only a few cells preserve a plasmacytoid appearance

Fig. 4.55
Cytology of undifferentiated cutaneous plasma cell tumours: pleomorphic neoplastic cells with marked anisokaryosis

Fig. 4.56
Cytology of undifferentiated cutaneous plasma cell tumours: pleomorphic neoplastic cells, most are multinucleated. Note that the typical plasma cellular aspects are completely lost
In less than 10 % of plasma cell tumours, it is possible to detect an amorphous fibrillary eosinophilic material interposed between the neoplastic cells. This represents the amyloid secreted by malignant plasma cells (Fig. 4.57) (Rowland et al. 1991). As the plasmacytoma is the only round cell tumour that produces amyloid, its presence allows the plasmacellular nature of the neoplastic tumour to be confirmed.
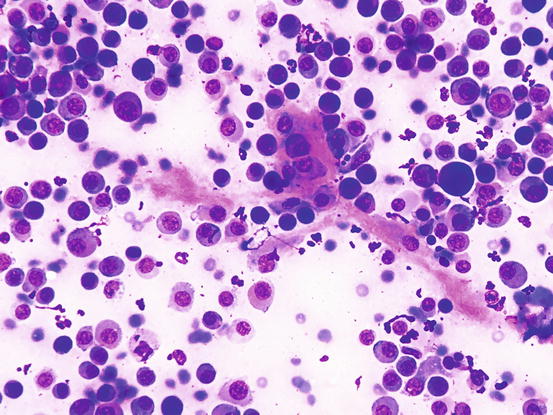

Fig. 4.57
Cytology of cutaneous plasma cell tumour: the eosinophilic amorphous material represents amyloid
Although amyloid is usually produced by better differentiated plasma cells, its presence can be helpful in suspect plasmacytoma when typical cytological aspects of plasma cell origin are lacking. To detect amyloid, slides must be stained with Congo red dye, which colours the amyloid red–orange (Fig. 4.58). In rare cases, the production of amyloid is so strong that it stimulates a massive granulomatous inflammation with numerous giant cells; in these cases the diagnosis of plasmacytoma can be very difficult.
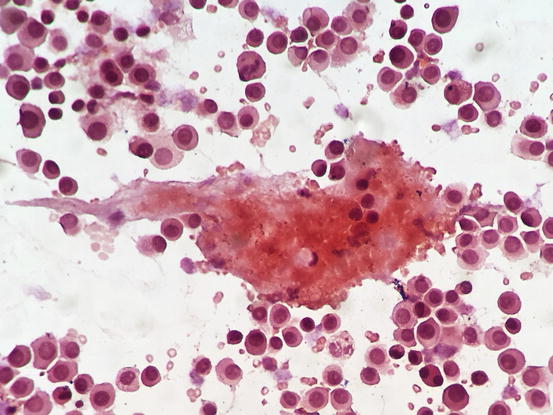

Fig. 4.58
Cytology of cutaneous plasma cell tumour: Congo red staining highlights the amyloid, as orange material intermingled between the neoplastic plasma cells
4.2.4 Transmissible Venereal Tumour
The canine transmissible venereal tumour (TVT) is a rare neoplasm of canidae, mostly reported in sub-tropical geographic areas (Das and Das 2000; Mukaratirwa and Gruys 2003). TVT cells have unique characteristics, showing a karyotype composed of only 59 chromosomes, very different from the 78 chromosomes of normal canine diploid cells. This tumour has the peculiarity of being transmitted from one dog to another, via the direct implantation of viable cells. It has been demonstrated that the ancestral TVT cell has been propagated over time, passing from animal to animal until the present day; in practice, the neoplastic cell behaves like a parasite (Cohen 1985; Murgia et al. 2006; Murchison et al. 2014).
Social and sexual behaviour and fighting between stray dogs are the basis of the location of the lesions, which, as mentioned above, result from direct implant of neoplastic cells. The skin lesions are mostly seen in immune-compromised or in stray dogs living in a poor state of health.
Lesions are single and of various sizes, from small nodules to very large erythematous masses, with irregular surfaces and of friable consistency, often ulcerated and bleeding. The classical lesions of TVT are mainly observed on the external genitalia mucosa (Figs. 4.59 and 4.60). In some dogs skin nodules can be observed, in association or not with genital lesions, varying in size, located on any part of the skin and often ulcerated. In stray dogs with only cutaneous nodules, an implantation secondary to bites during the fighting has been hypothesised. In dogs with multiple lesions, TVT cells are spread via the bloodstream or via lymphatics and these two ways of transmission justifies the metastases reported in different internal organs (Park et al. 2006). In one case report, TVT cells were detected in the peripheral blood in a severely immune-compromised dog that had developed hundreds of TVT nodules spread all over the body (Figs. 4.61 and 4.62) (Albanese et al. 2006).
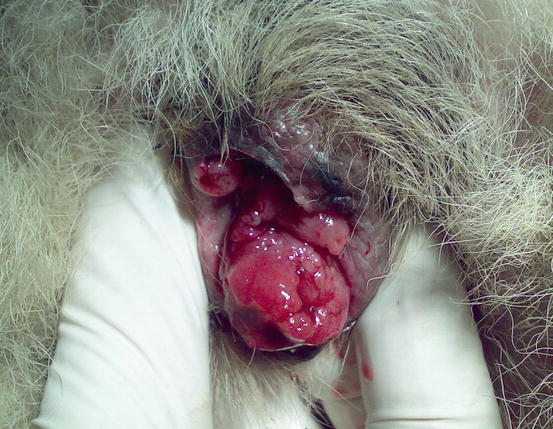
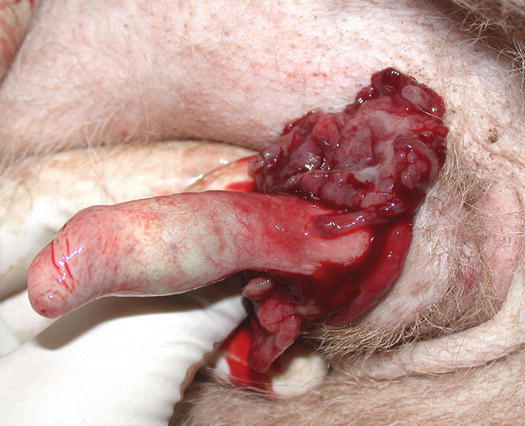
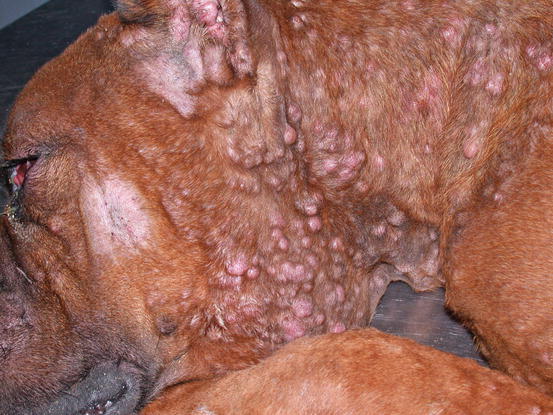

Fig. 4.59
Vulvar neoformation in a bitch with transmissible venereal tumour (TVT)

Fig. 4.60
Large, erythematous mass on the penis of a dog with TVT

Fig. 4.61
Multiple and confluent TVT nodules in an immune-compromised Boxer
Cytological Findings
The nature of TVT cells has been debated for many years. The latest theories seem to agree on their histiocytic nature, based on the demonstration of their phagocytic activity and on the positivity of several immunohistochemical markers to histiocytes (Mozos et al. 1996; Marchal et al. 1997; Albanese et al. 2002; Mukaratirwa and Gruys 2003; Gross et al. 2005; Park et al. 2006).
The cytological samples collected from TVT lesions are characterised by very high cellularity, with densely packed cells, which are sometimes difficult to identify as discrete round cells at low magnifications (Figs. 4.63 and 4.64).
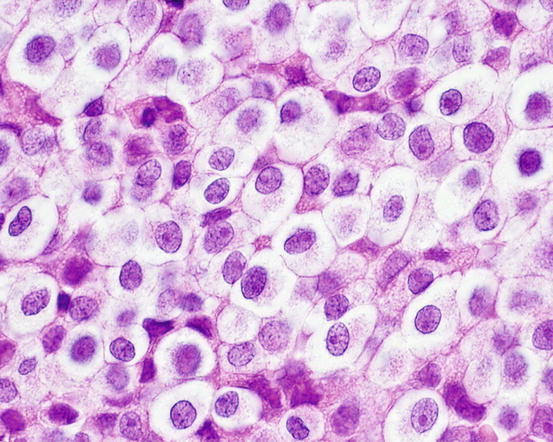
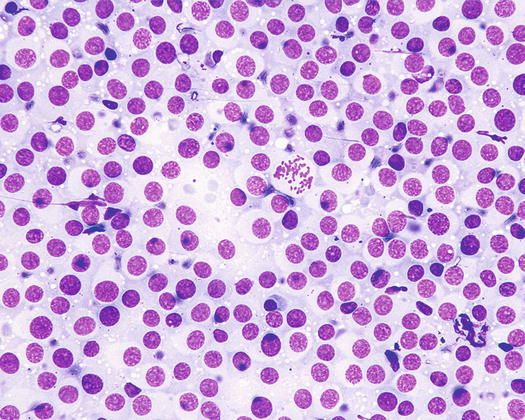

Fig. 4.63
Histology of TVT: discrete round cells with large cytoplasm. Note that with histology, the typical intracytoplasmic vacuoles are not as clearly evident as in cytological specimens

Fig. 4.64
Cytology of TVT: at low magnifications, the discrete arrangement of TVT cells is not easily recognisable
The nuclei are round, central or eccentric, with a regular chromatin pattern and a single nucleolus; the cytoplasm is large and pale and characterised by the presence of a variable number, usually high, of intracytoplasmic and non-overlapping vacuoles, of uniform size and with well-defined borders (Figs. 4.65 and 4.66). The anisocytosis is more obvious than in the other well-differentiated round cell tumours. Another characteristic histological feature of the TVT is the presence of a large number of mitotic cells, which must not be misinterpreted as an indicator of malignancy, as the TVT has a good prognosis owing to its excellent sensitivity to chemotherapy (Fig. 4.67). The TVT, although very rarely, can spontaneously regress following an attack of cytotoxic T-lymphocytes; therefore, the detection of a high amount of mature lymphocytes must be interpreted as a positive prognostic index.
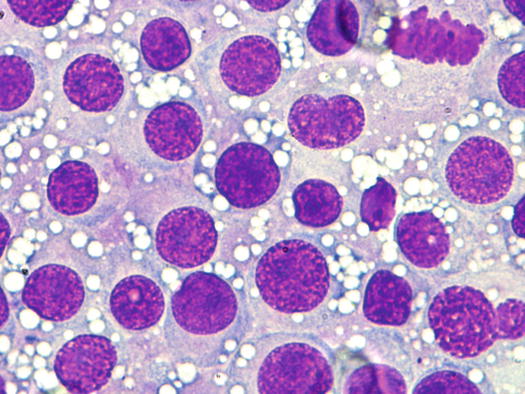
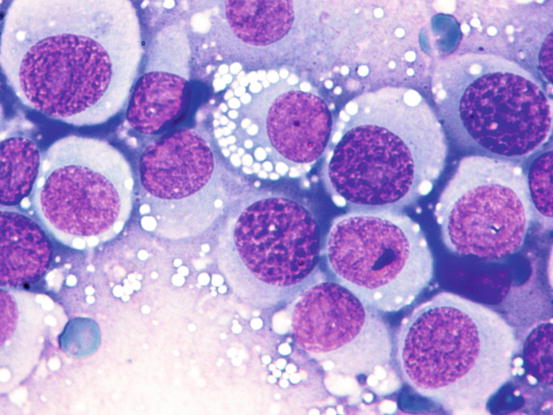
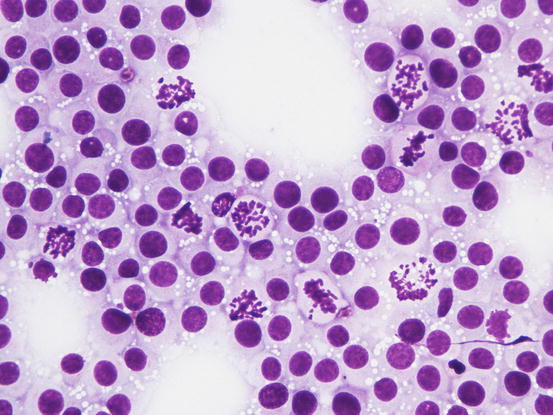

Fig. 4.65
Cytology of TVT: at medium magnifications, the round, well-delimited and non-overlapping vacuoles are clearly evident

Fig. 4.66
Cytology of TVT: at high magnifications, the round silhouette of TVT cells is undoubted

Fig. 4.67
Cytology of TVT: many mitotic cells characterise most TVT cytological slides
4.2.5 Histiocytic Diseases
Canine and feline histiocytic diseases encompass a complex group of reactive/neoplastic disorders, originating from dendritic cells, the so-called antigen-presenting cells (APCs), residing in the epidermis, dermis and subcutis.
This group includes the benign cutaneous histiocytoma, Langerhans cell histiocytosis, reactive histiocytosis (cutaneous and systemic) and the histiocytic sarcoma complex (localised and disseminated) (Moore 2014).
Some of the latter are not considered true neoplasms: histiocytoma is in fact a Langerhans cell proliferation that regresses spontaneously and reactive cutaneous histiocytosis is considered to be a reactive inflammatory response of interstitial dermal dendritic cells, based on the reported spontaneous regression of some cases and on the good response to several immune-modulatory drugs; for this reason, many authors prefer to group all these forms under the umbrella of histiocytic disorders or diseases and not as histiocytic neoplasia (Palmeiro et al. 2007; Moore 2014).
Although some of these disorders have some anamnestic, clinical and cytological peculiarities, differentiating them from other neoplastic and non-neoplastic diseases is not always easy.
A typical example is cutaneous reactive histiocytosis, which can be cytologically confused with other histiocytic diseases of macrophagic lineage, such as sterile granuloma syndrome or some nodular forms of leishmaniasis, in addition to the spindle variant of the histiocytic sarcoma, which cannot be cytologically differentiated from some anaplastic sarcomas of soft tissue with giant cells. In these cases, it is only possible to obtain a definitive diagnosis with the use of immunohistochemical investigations.
In cats, only the so-called feline dendritic progressive histiocytosis has been well documented as a true cutaneous histiocytic disorder; the few reported cases of histiocytic sarcoma in cats are mostly haemophagocytic (macrophagic lineage) and visceral in location (Affolter and Moore 2006).
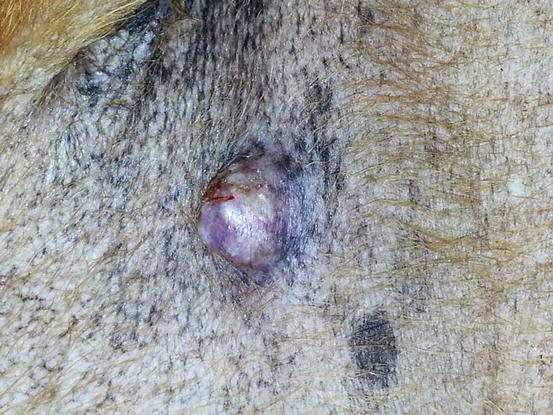
4.2.5.1 Benign Cutaneous Histiocytoma
The canine cutaneous histiocytoma is a benign proliferation of Langerhans cells, the APCs residing in the epidermis (Moore 2014). It is common in young dogs (less than 3 years of age), although it can be observed in dogs of all ages. It usually appears as a single nodule, localised mainly on the limbs, head and pinna, but it can grow in any part of the body. The canine histiocytoma usually has a domed or button-like shape, it can be alopecic with an erythematous surface and grows rapidly (Figs. 4.68, 4.69, and 4.70). Less frequently, multiple nodules, especially in Shar-pei dogs, are reported (Fig. 4.71) (Maina et al. 2014).
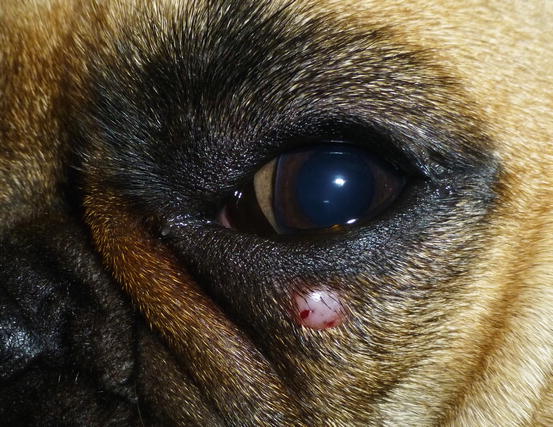
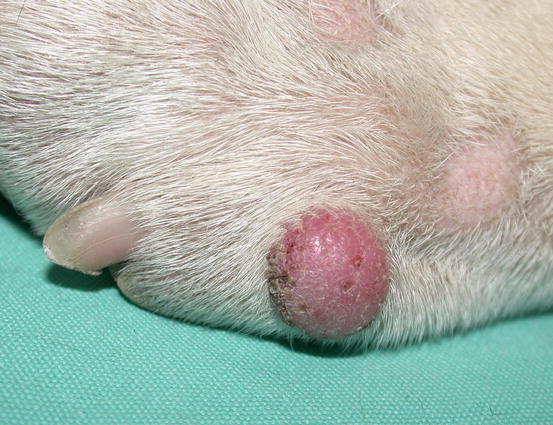
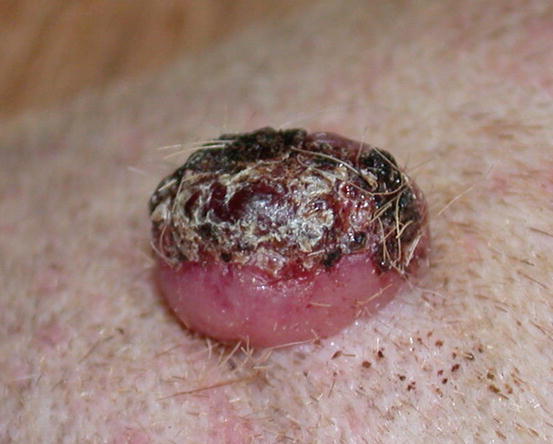
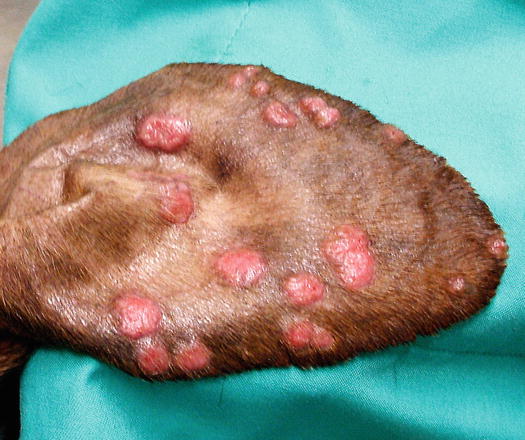

Fig. 4.68
Canine benign histiocytoma: small erythematous nodule on the eyelid of a French Bulldog

Fig. 4.69
Canine benign histiocytoma: erythematous and alopecic nodule on a digit of an English Bulldog

Fig. 4.70
Button-like alopecic, erythematous, ulcerated and crusty nodule, diagnosed as benign cutaneous histiocytoma

Fig. 4.71
Multiple cutaneous histiocytoma on the inner surface of the pinna of a dog (Courtesy of Dr. F. Leone, Italy)
Although cutaneous histiocytoma displays clinical features that can alarm many owners, such as the rapid growth and tendency to ulcerate, it is characterised by a benign biological behaviour that results in spontaneous regression within a few months (Fig. 4.72). In rare cases, single or multiple histiocytomas do not spontaneously regress and can persist for a long time (persistent cutaneous histiocytomas; Figs. 4.73 and 4.74) (Maina et al. 2014). Some Langerhans cells can migrate to lymph nodes, causing a secondary enlargement; these forms also tend to regress spontaneously. The presence of multiple cutaneous histiocytomas with dissemination of Langerhans cells in the lymph nodes and internal organs, characterises progressive Langerhans cell histiocytosis, a disorder that is usually fatal in affected animals (Nagata et al. 2000).
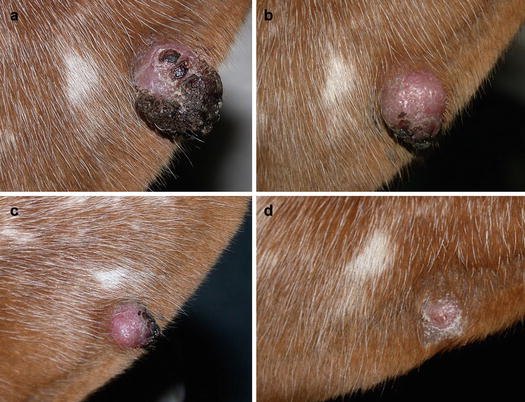
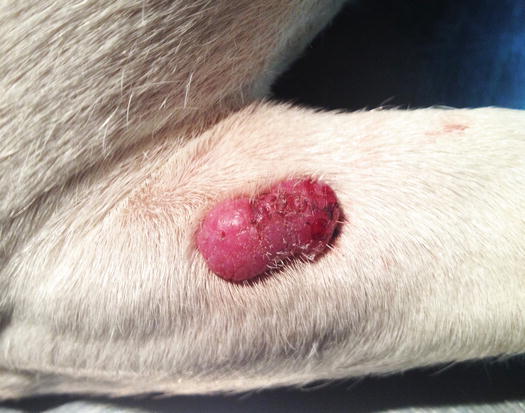
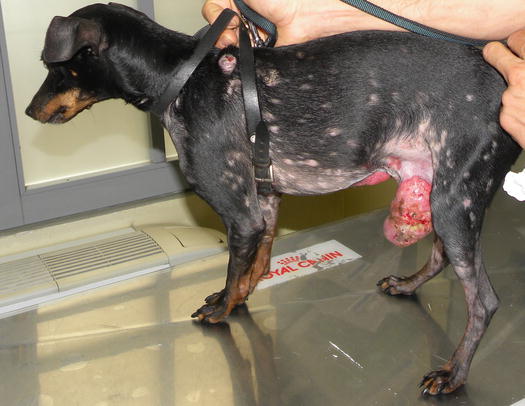

Fig. 4.72
Benign cutaneous histiocytoma. Regression phase: (a) day 0; (b) day 40; (c) day 60; (d) day 80

Fig. 4.73
Persistent cutaneous histiocytoma. The nodule was removed from the cytological diagnosis after 8 months. A histiocytoma was histopathologically confirmed

Fig. 4.74
Multiple nodules and masses spread all over the body of a Pinscher with multiple cutaneous histiocytomas (Courtesy of Dr. E. Maina, Italy)
Cytological Findings
Slides from histiocytoma are always highly cellular and are characterised by round discrete cells with slight anisocytosis and with a central or eccentric, round or oval nucleus, which often have a deep indentation that gives the nuclei a characteristic kidney shape; the chromatin is regular or stippled and the nucleolus, when visible, is small. The cytoplasm is from moderate to abundant and slightly basophilic or pale grey (Figs. 4.75, 4.76, and 4.77). In some cases, the cells are less characteristic and smaller, with scarce and more basophilic cytoplasm, creating difficulties in differentiating between lymphocytes and plasma cells (Fig. 4.78).
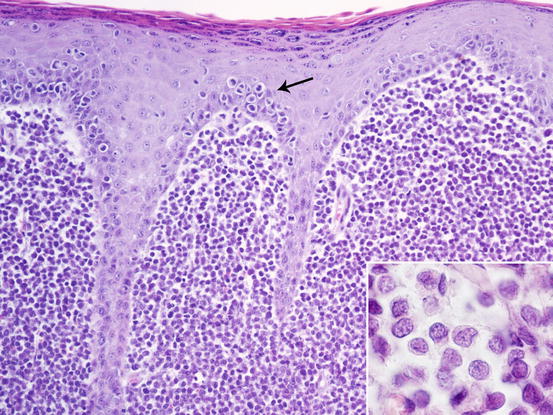
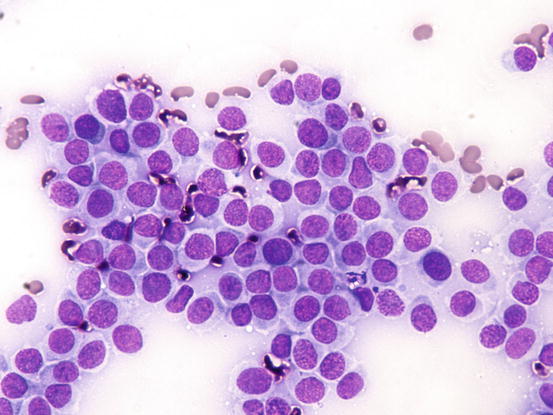
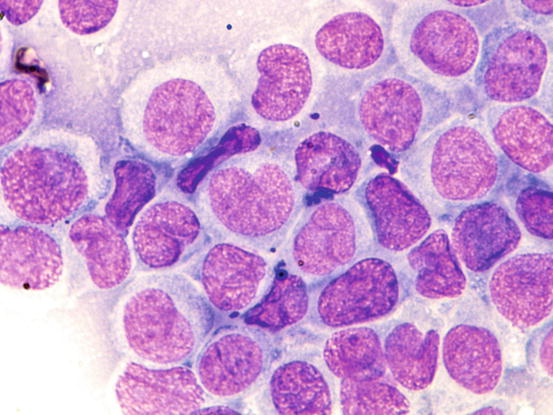
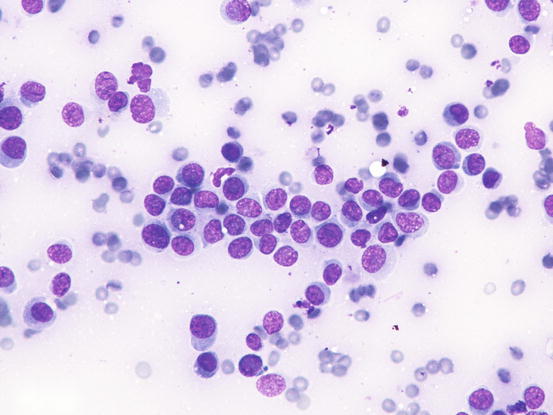

Fig. 4.75
Histopathology of cutaneous histiocytoma. Diffuse dermal proliferation of round and discrete cells. Note the epitheliotropism of cells (arrow) and the deeply folded nuclei (inset)

Fig. 4.76
Cytology of cutaneous histiocytoma. Many round cells with round and mostly central nuclei and with large pale blue cytoplasm

Fig. 4.77
Cytology of cutaneous histiocytoma. At high magnifications, the morphology of cells is more evident. Note the characteristic kidney-shaped nuclei

Fig. 4.78
Cytology of cutaneous histiocytoma. Small Langerhans cells with scarce cytoplasm can cause the misdiagnosis of plasma cell tumours
The cytoplasm may contain a low number of vacuoles even if, rarely, they can be numerous (Fig. 4.79). When specimens were collected in the regression phase, they are characterised by a variable number of small T-lymphocytes, which can be so numerous that they exceed the number of histiocytoma cells; the cytological sampling performed at this stage could make it difficult to diagnose the histiocytoma and may confuse the lesion for a chronic lympho-histiocytic inflammatory process (Figs. 4.80 and 4.81). In the advanced stages, together with lymphocytes, it is possible to find other histiocytic cells that are larger than those of a histiocytoma. These cells are characterised by a large cytoplasm and indented nuclei and represent infiltrating dendritic cells in the dermis. Finally, together with lymphocytes, a variable number of plasma cells can be observed. Some authors consider their presence a poor prognostic sign, which is observed in persistent histiocytomas. This biological behaviour linked to the presence of plasma cells does not seem to be based on any scientific evidence.
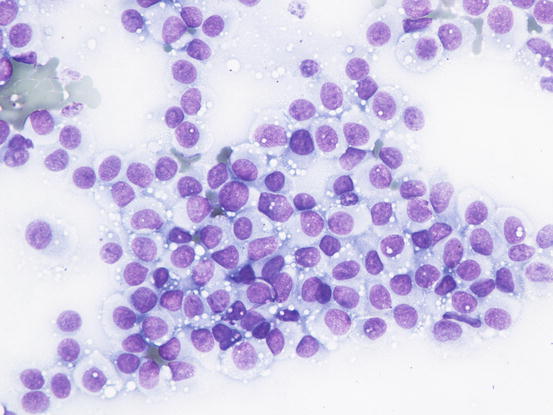
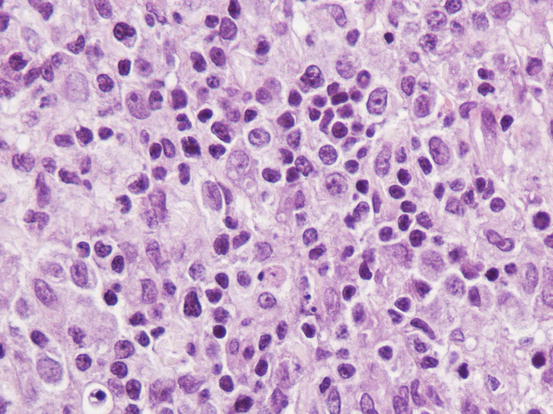
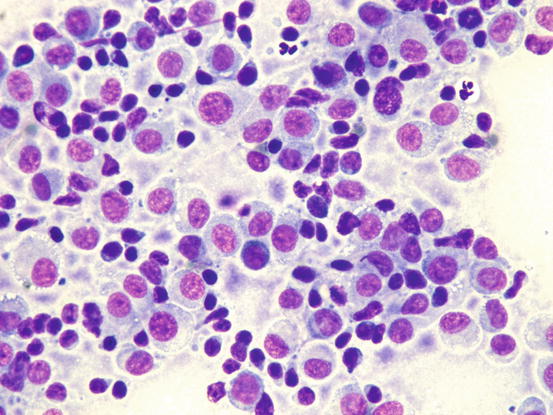

Fig. 4.79
Cytology of cutaneous histiocytoma. Many intracytoplasmic vacuoles are clearly evident. Note that the cells resemble TVT cells

Fig. 4.80
Histopathology of cutaneous histiocytoma in regression stage. Many small infiltrating lymphocytes infiltrate the tumour

Fig. 4.81
Cytology of cutaneous histiocytoma in the regression phase. Many small lymphocytes are detectable between the dendritic cells of the histiocytoma
4.2.5.2 Cutaneous and Systemic Reactive Histiocytosis
Cutaneous reactive histiocytosis (CH) is an inflammatory lympho-histiocytic proliferation of the dendritic APCs residing in the dermis; a probable deregulation of the immune system of unknown origin, probably arising from the defective interaction of dendritic cells and T cells is suspected (Moore 2014). This pathogenic hypothesis is based on the spontaneous resolution of the lesions and the good response to several immune-modulatory drugs observed in many cases.
Two forms of reactive histiocytosis are reported: the cutaneous, in which lesions are confined to the skin, and another form characterised by the simultaneous involvement of skin and internal organs, named reactive systemic histiocytosis (SH) (Moore 1984, 2014; Affolter and Moore 2000). Not all cases of SH, however, have skin lesions. Skin lesions are represented by multiple nodules or plaques, dermal and subcutaneous in location, of variable size and appearance, sometimes showing a donut or an arc shape, which look very similar to the lesions observed in dogs with non-epitheliotropic lymphoma; the nodules are mostly localised on the head, face, trunk, scrotum, nose and extremities (Figs. 4.82, 4.83, 4.84, and 4.85). Many dogs simultaneously present involvement of the conjunctival and nasal mucosa. In the latter case, the nose can assume a so-called clown nose appearance (Gross et al. 2005). Sporadic cases exclusively localised to the nose are reported. In some cases, multiple nodules are arranged in a linear fashion to suggest distribution along the blood and lymphatic vessels (Fig. 4.86).
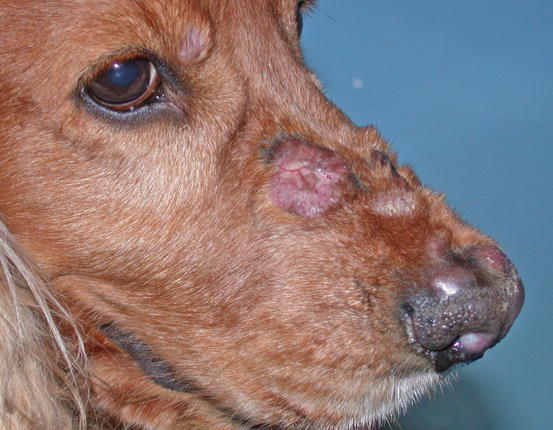
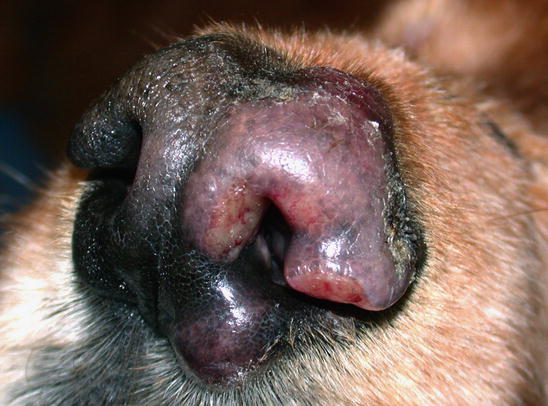
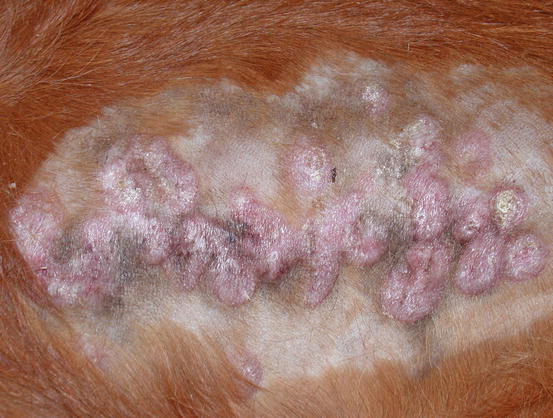

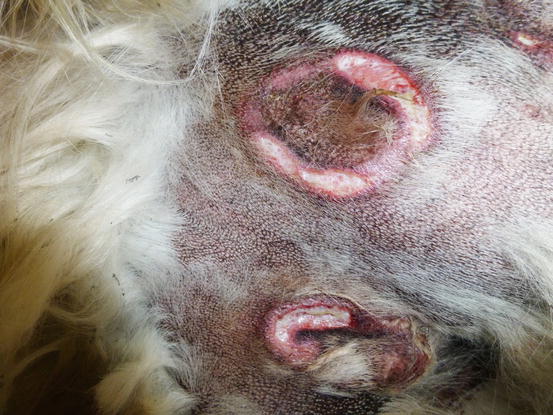

Fig. 4.82
Nodules and donut-shaped plaques in a Cocker with reactive cutaneous histiocytosis

Fig. 4.83
Reactive cutaneous histiocytosis: nodule and swelling of the nose in the same dog as in Fig. 4.82

Fig. 4.84
Multiple and confluent donut-shaped plaque on the chest of a dog with reactive cutaneous histiocytosis

Fig. 4.85
Multiple erythematous nodules on the thorax of a Bernese Mountain dog with cutaneous reactive histiocytosis

Fig. 4.86
Reactive cutaneous histiocytosis. Large annular ulcerated plaques on the sternal area of a Golden retriever
Cytological Findings
The cytological features depend on the stage of the disease. Histologically, reactive histiocytosis is characterised by peri-adnexal and often angiocentric granulomas, located in the deep dermis (bottom heavy) and infiltrating the panniculus (Figs. 4.87 and 4.88). In early stages, via FNB, the possibility of the needle penetrating the granulomas is very low; thus, samples usually contain few cells. In the advanced stages of the disease, granulomas can merge and the infiltrate changes from nodular to diffuse, allowing more successful collection of cells. In many cases, cells are yielded in a cohesive group and it is very hard to observe intact dendritic cells with a clear silhouette. More representative specimens are characterised by roundish histiocytes with oval, folded or kidney-shaped nuclei, regular chromatin, inconspicuous nucleoli and large and pale cytoplasm, sometimes with poorly defined margins and occasionally containing small vacuoles (Figs. 4.89 and 4.90). Many histiocytes are represented by bare nuclei with cytoplasm spread on the background. In association with histiocytic dendritic cells, a variable but usually high number of lymphocytes is constantly present; a few plasma cells and neutrophils are also detected. In most cases, cytological features are not differentiable from those observed in sterile granuloma syndrome or other granulomatous diseases, as the APCs can be cytologically similar to histiocytes of the macrophage lineage.
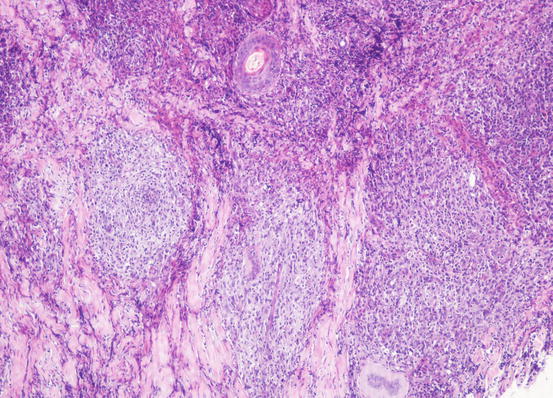
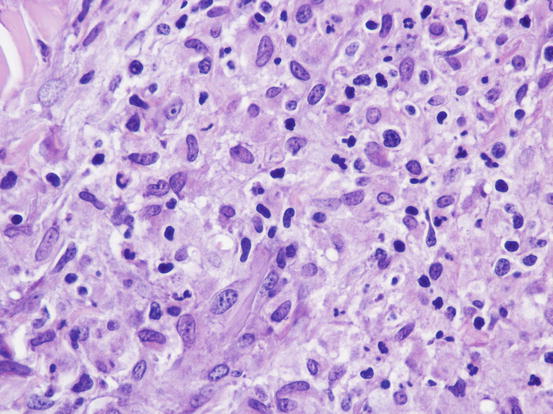
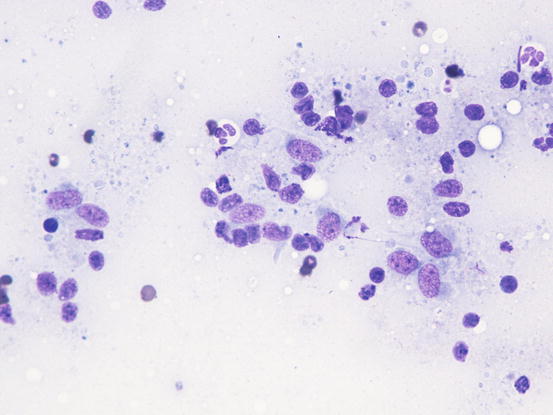
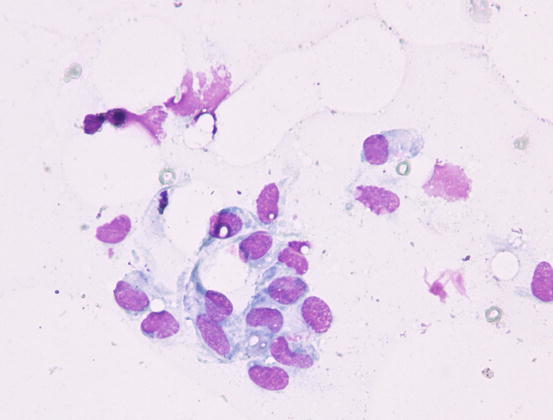

Fig. 4.87
Histopathology of reactive cutaneous histiocytosis: multiple deep and confluent dermal nodules

Fig. 4.88
Histopathology of reactive cutaneous histiocytosis: at high magnifications, large histiocytes and small lymphocytes are recognisable

Fig. 4.89
Cytology of reactive cutaneous histiocytosis: histiocytes with oval and slightly indented nuclei. Many free bare nuclei and a few lymphocytes are also present

Fig. 4.90
Cytology of reactive cutaneous histiocytosis: histiocytes with oval and kidney-shaped nuclei, with poorly defined cytoplasmic margins
Definitive diagnosis, in cases where there is doubt, can only be achieved by histopathology and immunohistochemistry on frozen tissue, as on paraffin-embedded tissue, there is no one immunostain that can differentiate a dendritic histiocyte (APC) from a macrophage.
4.2.5.3 Histiocytic Sarcoma
Histiocytic sarcoma is a rare neoplasia in dogs and very rare in cats, and originates from interstitial dendritic cells present in many organs (Affolter and Moore 2002; Moore 2014). In the skin, the APCs from which the histiocytic sarcoma originates are present in the dermis and subcutaneous tissue.
The primarily cutaneous form is observed more frequently in certain breeds such as the Bernese mountain dog, the Rottweiler, the Golden and Flat-coated retriever (Moore and Rosin 1986; Constantino-Casas et al. 2011; Moore 2014) as single nodules of variable size, predominantly localised to the extremities, especially on the joints area, which are very often infiltrated (Fig. 4.91). The same neoplasia can metastasise to the internal organs or may originate primarily from a visceral organ such as the spleen. A clinical variant of histiocytic sarcoma that started as disseminated in multiple visceral organs and that can involve even the skin is well recognised (Figs. 4.92 and 4.93). All these forms with multicentric lesions are defined by the term disseminated histiocytic sarcoma, supplanting the old term malignant histiocytosis.
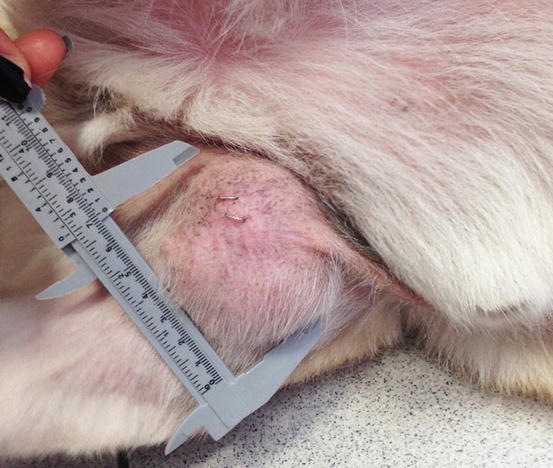
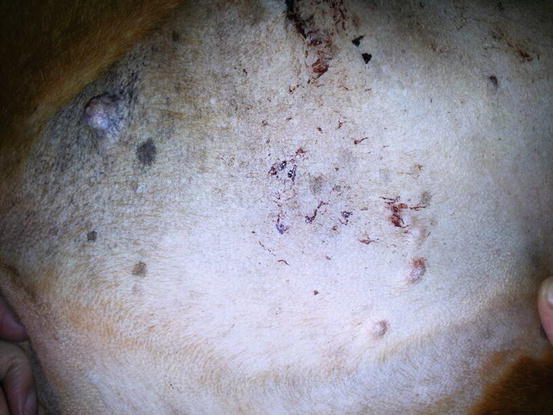

Fig. 4.91
Single mass on the leg of a dog with localised histiocytic sarcoma (Courtesy of Dr. L. Marconato, Italy)

Fig. 4.92
Multiple small nodules in a boxer with disseminated histiocytic sarcoma
Cytological Findings
The cytological pattern of histiocytic sarcoma is pleomorphic and may vary depending on the area of the lesion that is sampled. It is possible to find two different types: a variant composed prevalently of round cells and another form with spindle-shaped cells. In any case, the malignant aspects of the neoplastic cells are always remarkable. The round cells are characterised by marked anisocytosis, with very large, round or oval, often indented or kidney-shaped nuclei, with one or more bizarre nucleoli and large cytoplasm, slightly basophilic and usually micro-vacuolated (Figs. 4.94 and 4.95). The spindle cells have round to oval, often indented and distorted nuclei. In both variants, the cells exhibit severe aspects of atypia and in the spindle cell subtype, the presence of numerous multinucleated cells can create many diagnostic difficulties as the cytological features are very similar to those observed in some anaplastic sarcomas of the soft tissue with many giant cells (formerly named malignant fibrous histiocytoma; Figs. 4.96 and 4.97). In some samples it is also possible to find a variable number, often significant, of infiltrating leukocytes.
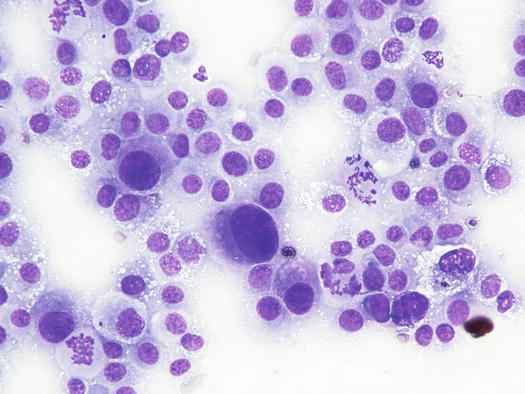
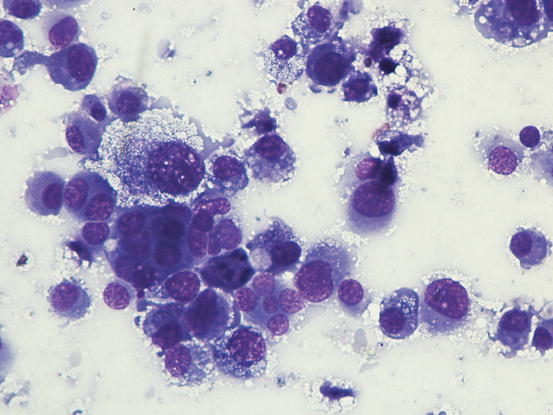
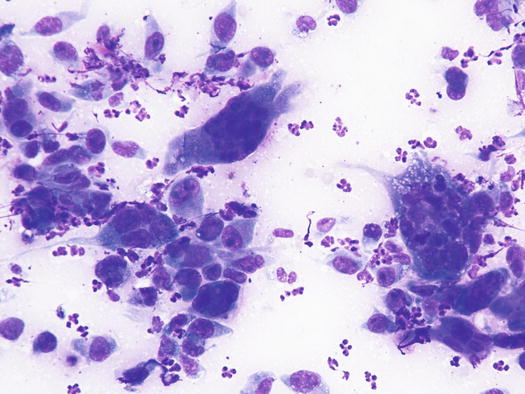
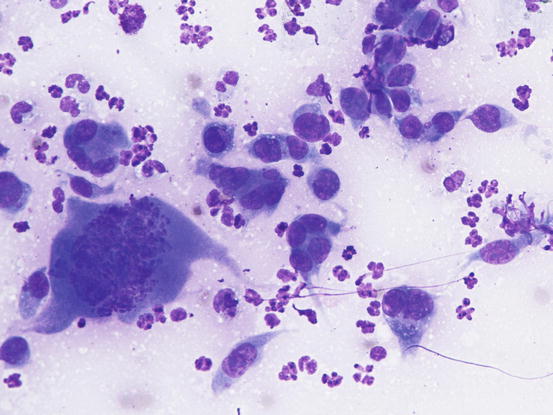

Fig. 4.94
Cytology of histiocytic sarcoma: round neoplastic dendritic cells, characterised by severe anisokaryosis and vacuolated cytoplasm

Fig. 4.95
Cytology of histiocytic sarcoma: round neoplastic dendritic cells, characterised by severe atypia and multinuclearity

Fig. 4.96
Cytology of histiocytic sarcoma: spindle-shaped neoplastic dendritic cells. Marked atypia and multinuclearity characterise the cells. Note as they cannot be cytologically differentiated from a spindle-shaped anaplastic soft tissue sarcoma

Fig. 4.97
Cytology of histiocytic sarcoma: note the severe atypical mitosis in the larger cell
4.2.5.4 Feline Progressive Dendritic Histiocytosis
Feline progressive histiocytosis is a histiocytic disorder with involvement of the skin, observed in adult to old cats and originating from interstitial APCs (Moore 2014; Pinto da Cunha et al. 2014). In most reported cases, lesions are usually characterised by a single nodule on the limb that with time disseminates to other skin sites (Figs. 4.98, 4.99, and 4.100). The spontaneous regression of some nodules is sometimes observed, but the disease always progresses and spreads to the skin or the internal organs. The cutaneous nodules vary in size, from small to very large and may coalesce in plaque formations mostly located on the head, limbs and trunk.
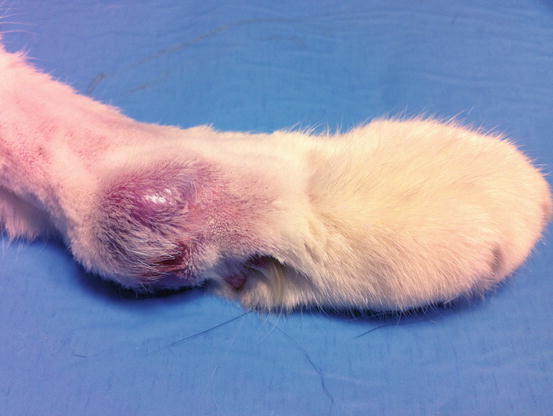
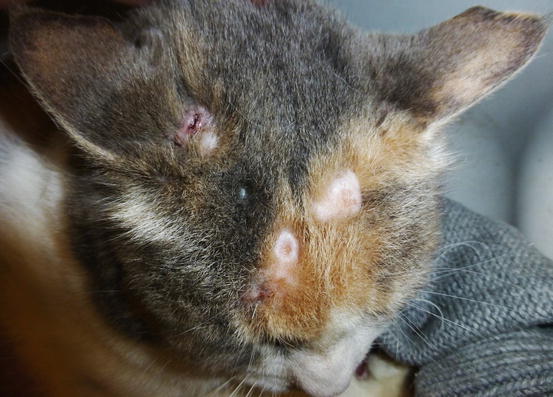
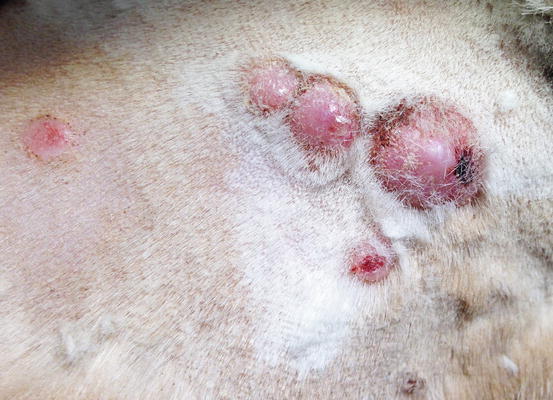

Fig. 4.98
Feline progressive histiocytosis. Single, alopecic and erythematous nodule on the leg

Fig. 4.99
Multiple alopecic nodules on the head of a cat with progressive histiocytosis

Fig. 4.100
Feline progressive histiocytosis. Close-up of multiple skin nodules on the chest of the same cat as in Fig. 4.99
Cytological Findings
Slides from feline progressive histiocytosis are variable. In the initial stage, histiocytes vary from round to polygonal and less frequently spindle-shaped, with nuclei from round to indented, sometimes double and rarely multiple with scant cytoplasm stained pale blue (Figs. 4.101 and 4.102). In chronic cases the cytological atypia become much more evident until they become very pronounced (anisokaryosis, anisocytosis, bizarre nuclei, large and multiple macronucleoli) (Figs. 4.103 and 4.104). In chronic cases, a significant infiltration of lymphocytes and, less frequently of mast cells, is reported. Because of the difficulty of diagnosing the APCs in paraffin specimens that restrict the use of immunohistochemical markers, the possibility of using immunocytochemistry on frozen cytological specimens has recently been suggested in some works. The use of specific markers for the identification of various populations of dendritic histiocytes permits us to differentiate them not only from other dendritic cells, but also from histiocytic macrophages (Pinto da Cunha et al. 2014).
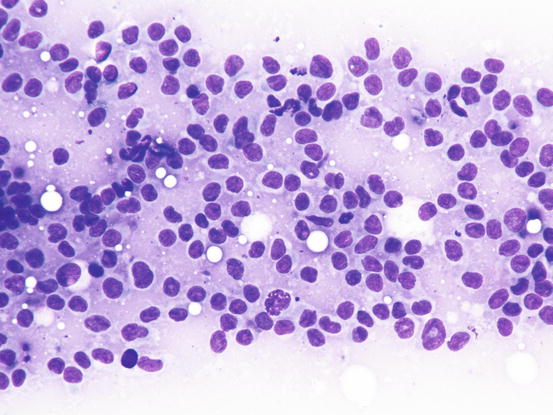
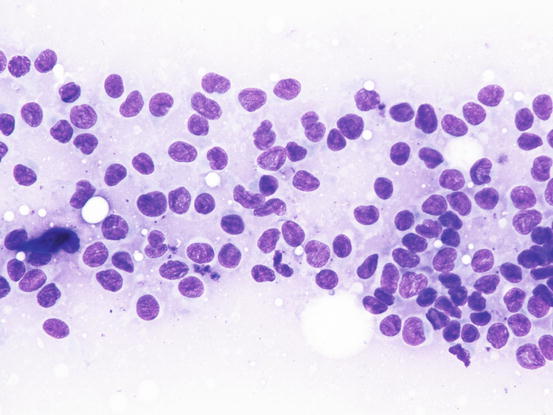
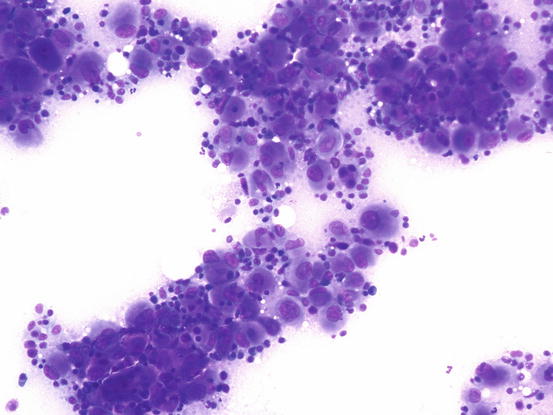
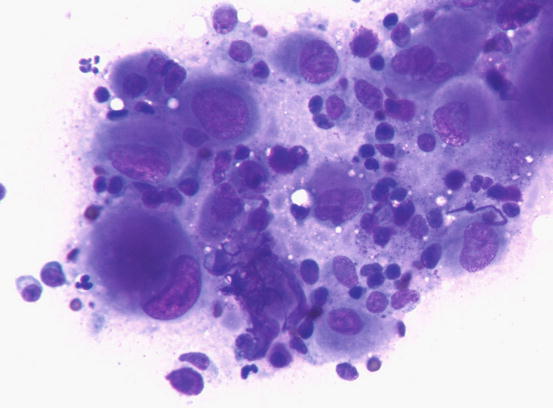

Fig. 4.101
Cytology of feline progressive histiocytosis. Discrete round dendritic antigen-presenting cells (APCs)

Fig. 4.102
Cytology of feline progressive histiocytosis. At high magnification, the histiocytic features are more evident. Note that many cells show indented nuclei

Fig. 4.103
Cytology of feline progressive histiocytosis. Highly cellular specimens composed of polymorphous round cells with severe malignant features

Fig. 4.104
Cytology of feline progressive histiocytosis. At high magnifications, large histiocytic dendritic cells of different sizes and some lymphocytes are evident
4.3 Epithelial Tumours
In this sub-group, tumours that originate from the epidermis and its adnexa are discussed.
Epithelial cells present in the skin, from where neoplasia can originate, are the keratinocytes from both external and follicular epidermis, and sebaceous and apocrine glands.
In addition to these tumours, neoplasms of specialised glands, which can be both apocrine (ceruminous and anal sac glands) and sebaceous (hepatoid glands) are also discussed.
The cytological features common to all epithelial tumours is that cells are yielded as cohesive clusters, as they are, by nature, strictly connected to each other by intercellular joints.
The epithelial tumours are composed of medium to large cells, with scarce to abundant cytoplasm and round to ovoid nuclei with chromatin ranging from fine to coarse. The presence of acinar, ductal and papillary cytoarchitecture, together with intracytoplasmic secretions, can cytologically identify the apocrine glandular origin of the tumour. Lobules of cohesive cells with foamy cytoplasm are instead indicative of their sebaceous origin.
In poorly differentiated tumours (anaplastic carcinomas), the above mentioned cytological features may be lost and the cells cannot be released as clusters, but singly and with severe cytological atypia. In these cases, the morphological aspects may make it difficult to include the tumour in the group of epithelial tumours. A classic epithelial tumour, in which the cells often appear singly, is the squamous cell carcinoma.
The epithelial neoplasms of the skin in dogs and cats are numerous and only the most important, or those for which the cytological examination is of great diagnostic aid, are described in detail in this chapter.
4.3.1 Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is a very common cancer in cats, but less common in dogs, and originates from the cells of the spinous layer of the epidermis (Gross et al. 2005; Webb et al. 2009; Hauck 2013; Murphy 2013). The main cause of SCC in pets is linked to chronic sun exposure and therefore SCCs are very frequent in animals with white skin, especially on glabrous or poorly haired areas and with thin skin.
The role of papillomavirus in the development of SCC has been widely documented in dogs and the ongoing discovery of new viral variants is a constant confirmation (Zaugg et al. 2005). In cats, the role of papillomavirus is limited to a few reports, although recent studies have shown that in many cases of SCC and Bowenoid in situ carcinoma (BISC), papillomavirus particles have been detected (Nespeca et al. 2006; Wilhelm et al. 2006; Favrot et al. 2009). Moreover, although BISC may arise on feline viral plaques induced by papillomavirus (papillomavirus-induced feline viral plaque), some cases may also be associated with a non-viral cause (Wilhelm et al. 2006).
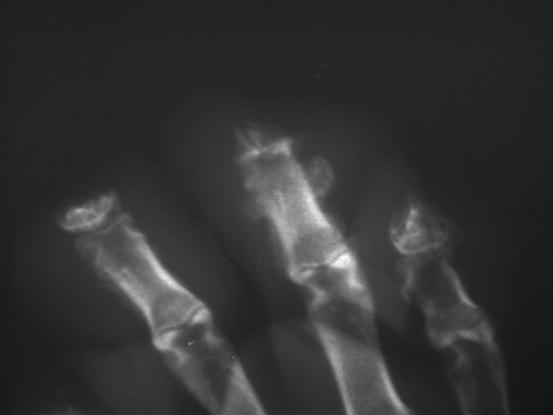
In dogs, the SCCs appear as nodules, plaques or exophytic masses with ill-defined margins and often with an ulcerated and bleeding surface. These tumours are mainly located on hairless and thin skin, such as the groin, abdomen and scrotum, especially in dogs with white skin, which is less protected from sunlight (Figs. 4.105 and 4.106). In many dogs, lesions are observed in association with actinic keratosis, which represents an in situ carcinoma that precedes the development of an infiltrating SCC (Fig. 4.107). Ulcerative SCCs are also observed on the nose, particularly in Labradors and Golden retrievers (Fig. 4.108) (Lascelles et al. 2000). Another area where SCC develops in dogs, considered highly malignant, is the nail bed; this location is most commonly observed in black-haired giant breeds such as in giant Schnauzers, Rottweilers and Labradors, where multiple toes of more than one leg can be simultaneously affected (Figs. 4.109 and 4.110). This must be stressed, as in the early stages of tumour development, lesions are characterised by the swelling of the digit and periungual area and cannot be clinically differentiated from inflammatory paronychia.
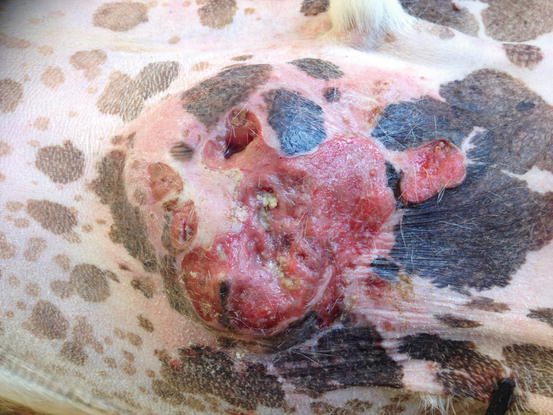
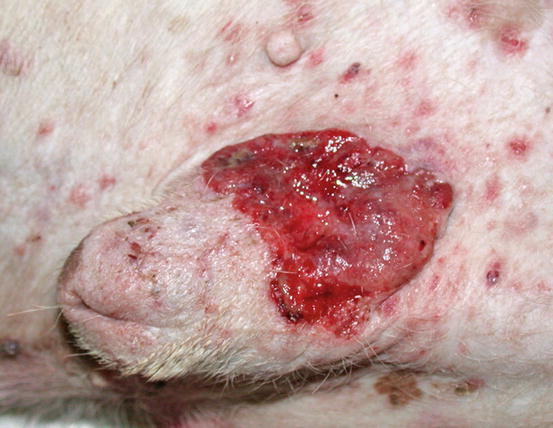
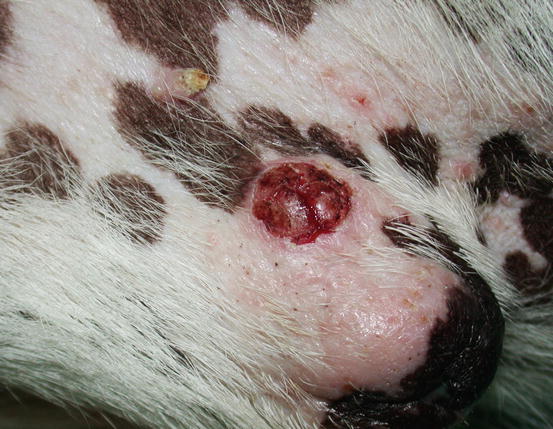
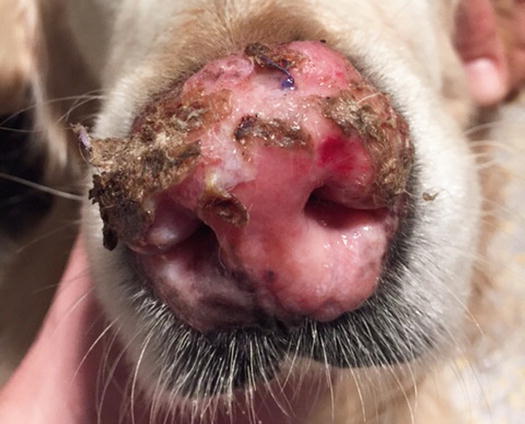
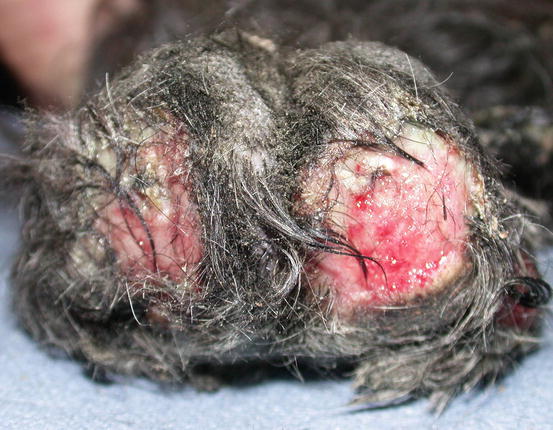

Fig. 4.105
Squamous cell carcinoma (SCC). Large and ulcerated mass on the abdomen of an Argentine dog

Fig. 4.106
Squamous cell carcinoma. Ulcerated SCC on the penis of a dog

Fig. 4.107
An SCC and actinic keratosis on the penis of a white hair-coated dog

Fig. 4.108
An SCC of the nose in a Golden retriever (Courtesy of Dr. M. Annoni, Italy)

Fig. 4.109
Multidigital SCC of the nail bed in a Giant Schnauzer
In cats, SCC is frequently observed on the nose, eyelids and on the pinna. Even in this species, the white coat is a strong predisposing factor. In the cat, the SCC occurs more frequently with ulcerative lesions that cause the loss of large portions of tissue disfiguring the affected areas (Figs. 4.111, 4.112, 4.113, and 4.114). Exophytic lesions are much less common than in dogs (Fig. 4.115).
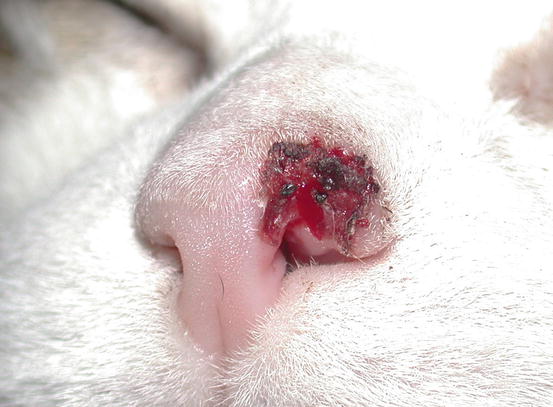
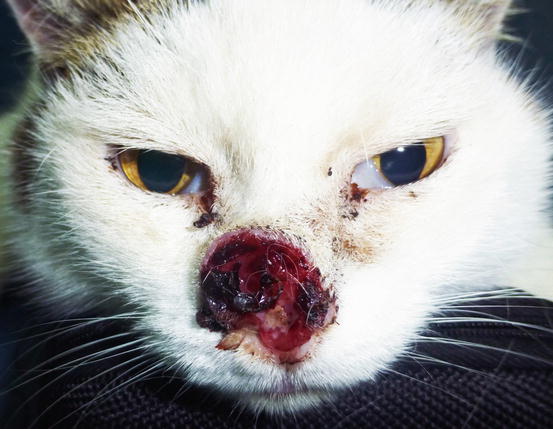
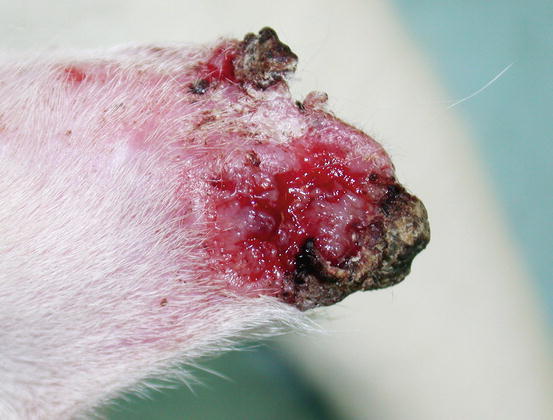
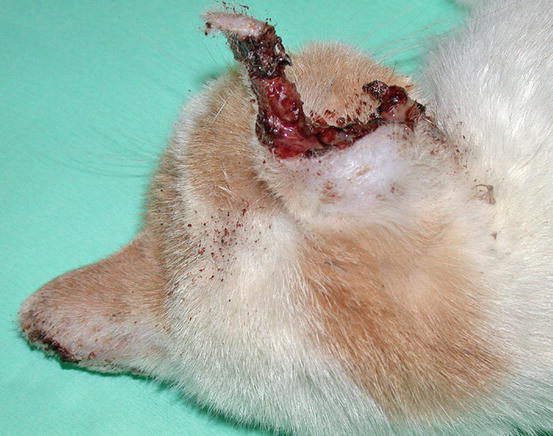
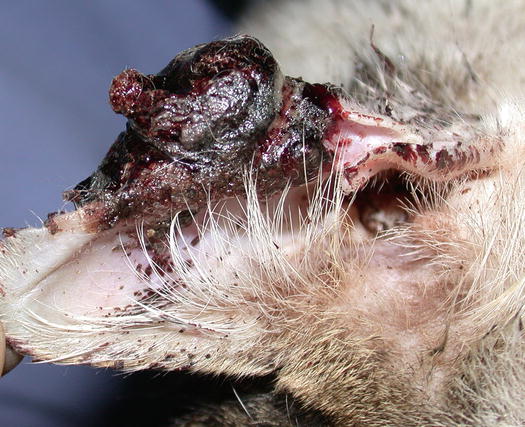

Fig. 4.111
An SCC in a cat. Ulcerated, bleeding and crusty lesion affects the nose

Fig. 4.112
White-haired cat with severe and deforming SCC of the nose

Fig. 4.113
Ulcerated SCC on the pinna of a white-haired cat

Fig. 4.114
Typical aspect of the SCC in a cat. Ulceration of both ear pinnas

Fig. 4.115
Nodular SCC exophytic in growth on the pinna of a cat
Cases of non-sun-induced SCCs are usually observed, as single or multiple lesions, in cats with dark coats (BISC). In cats affected by BISC, skin lesions are usually characterised by multiple pigmented plaques, often covered with thick yellowish or brownish crusts, spread throughout the body and with frequent facial involvement. Clinically, they are often not suspected to be neoplasia, but are confused with non-tumoral lesions (Figs. 4.116, 4.117, and 4.118).
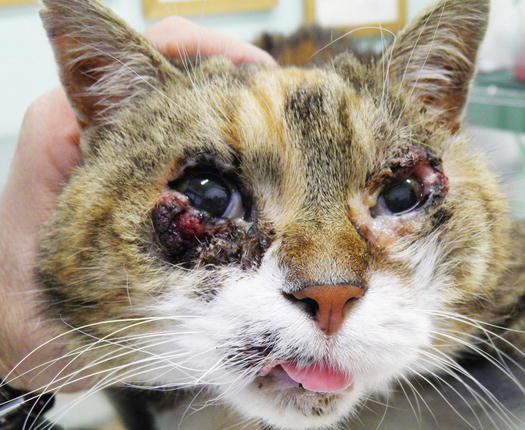
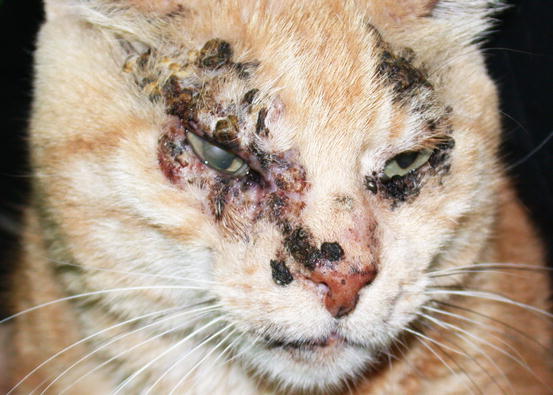
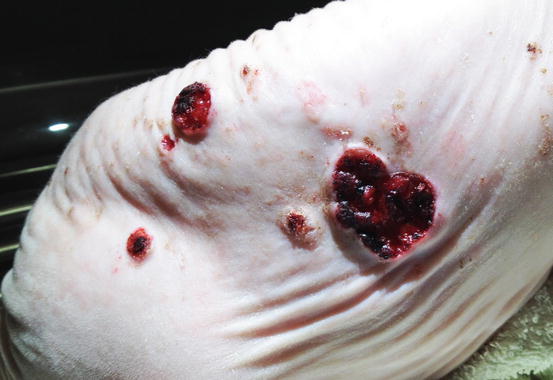

Fig. 4.116
In situ carcinoma on the eyelids of a cat

Fig. 4.117
In situ carcinoma presented as multiple dark crusty plaques on the face

Fig. 4.118
Multiple lesions of both in situ carcinoma and SCC on the trunk of a Sphynx cat
Cytological Findings
The cytological features of SCC is linked to its grade of differentiation and different aspects can coexist within the same neoplasia. Very pleomorphic features characterise the cytological specimens (Figs. 4.119 and 4.120). In most instances, the SCCs are well-differentiated and are cytologically characterised by small aggregates, more or less numerous, of epithelial cells in which evident aspects of squamous differentiation are present. Although the SCC is an epithelial neoplasia, the cells tend not to always maintain real cohesion and, for this reason, they are frequently yielded as single cells (Fig. 4.121).
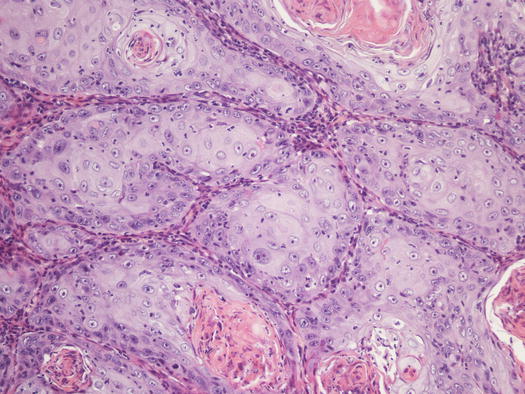
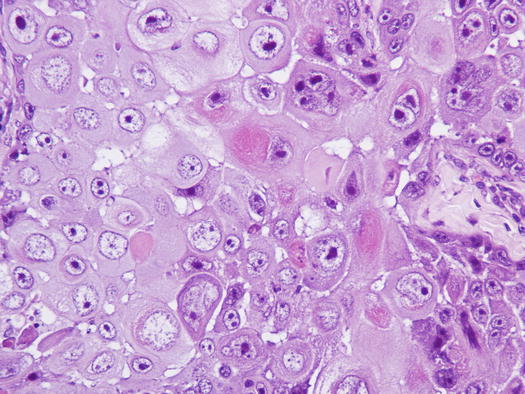
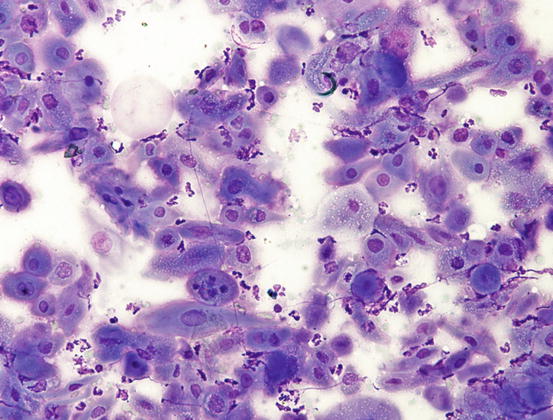

Fig. 4.119
Histopathology of SCC. Multiple nests of squamous neoplastic cells. Note the production of many keratin pearls

Fig. 4.120
Histopathology of SCC. Anisocytosis, anisokaryosis and macronucleoli represent the malignant aspects of the neoplastic keratinocytes

Fig. 4.121
Cytology of SCC. Marked asynchronous maturation of neoplastic keratinocytes
The main cytological characteristic of SCC, already detectable at low magnifications, is the typical asynchronous maturation of different squamous cells and, between the nucleus and the cytoplasm in the context of the same cell: a reduction of the size of the nucleus does not always follow a squamous differentiation of cytoplasm. Squamous differentiation manifests with the polygonal to roundish shape of the cells, with a large cytoplasm, which show very different colours, from light blue to greyish to pink (Fig. 4.122). In poorly differentiated SCCs, the diagnosis can be difficult, as very few cells exhibit squamous differentiation and slides may present more or poorly cohesive clusters of neoplastic keratinocytes with a high N/C ratio without any obvious squamous differentiation. However, some cells with squamous features are usually detected in most undifferentiated SCCs (Figs. 4.123 and 4.124).