Diagnosis requires a full, detailed history to be taken as well as a full health examination. A diagnosis of a behavioural condition can only be made accurate by extensive history taking and elimination of medical conditions from the picture.
Behavioural feather plucking
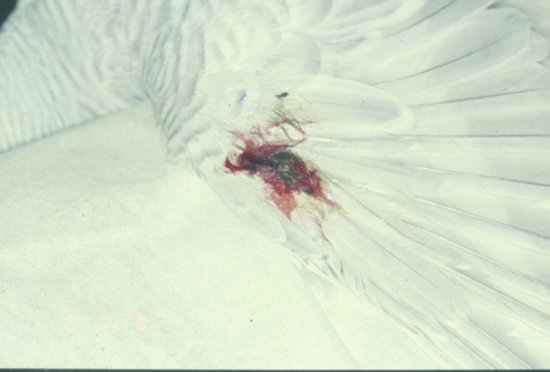
The most basic classification is to divide feather pluckers into two types. The first is the true feather plucker which removes the whole feather. The second is the feather chewer which just mutilates the feather but leaves it embedded in the skin. In addition, some species of Psittaciform bird such as the cockatoos may also exhibit self-mutilation of the body as well as feather plucking.
The condition is mainly seen in members of the Psittaciformes, although some of the hawk family such as Harris’ Hawk are also susceptible.
Behavioural feather plucking may be diagnosed often only after the ruling out of infectious/pain causes.
Ectoparasites
Ectoparasites are not as commonly seen in cage birds as might be expected. There are, however, many important skin and feather parasites of raptors, game birds and waterfowl kept in captivity.
Mites
Knemidocoptes: They are seen mainly in the budgerigar and canary. These eat cell debris and cause the conditions known as ‘scaly beak’ and ‘tassle foot’. The presenting signs are crusting and enlargement of the cere at the base of the beak, and thickening and proliferation of the skin of the legs. Another mite peculiar to pigeons is the depluming mite (Knemidocoptes laevis). This mite causes disintegration of the feather quill, causing it to break off close to the skin and producing bald areas.
Sarcoptes
Scabies mites (Sarcoptes spp.) are uncommonly seen, but have been reported in macaws. The symptoms are feather loss and widespread self-trauma.
Dermanyssus
The red feather mite (Dermanyssus gallinae) has been reported in raptor flights, pigeon lofts and in some cases, cage and aviary birds such as parrots, particularly those in outside flights. This mite does not live on the bird permanently. It hides in the cracks and crevices of the cage or shelter during the day and crawls out to attack the bird when it is roosting at night. It is a blood sucking mite and can cause anaemia and weakness in heavily parasitised birds.
Ornithonyssus
Another blood sucking mite is the northern fowl mite (Ornithonyssus sylviarum). This mite inhabits the host continuously, so it is easier to detect and to treat.
Other mites such as the skin mites, Backericheyla spp. and Neocheyletiella media in passerine birds, and Epidermoptes bilobatus and Michrolichus avus may also cause pruritus. Quill mites such as Dermatoglyphus spp. and Syringophylus spp. may also cause pruritus, feather picking and loss.
Lice
All avian lice belong to the order Mallophaga, and so cause their damage by chewing the feathers. They are generally elongated, squeezing in between the barbs of the feathers. They are more commonly seen in outdoor-housed birds and the main route for infestation is from wild birds.
The waterfowl mite is Holomenopon spp. It has been associated with damage of feathers, leading to a lack of normal structure and loss of waterproofing. This causes the feathers to look bedraggled and soggy so-called ‘wet feather’ disease.
In pigeons, two main types of louse are seen. The body louse (Menapon latum) and the slender louse (Columbicula columbae) are seen on the wings. They cause damage to the structure of the feather, damaging the interlocking of the barbs which keep the feather’s shape intact, and so give the pigeon a somewhat ragged appearance.
Raptors are prone to lice and a wide variety of species-specific lice are found. The mites destroy feather integrity and this leads to poor flight.
Flies
The blow fly family, such as the blue, black and green bottles, may be drawn to birds with diarrhoea or with wounds. The maggots of these species then eat their way into the bird with devastating results, and allow the colonisation of the wounds by smaller species of fly maggot.
The Hippoposcidae genus contains such species as the sheep and horse keds, which are however capable of infesting aviary birds, particularly raptors. The juvenile form of the fly is flightless and so spends large amounts of time living on its host. These species will often create infection and anaemia. They can also transmit blood-borne parasites, e.g. avian malaria, Haemoproteus spp. and Leucocytozoan spp., which may cause anaemia and immunosuppression. The pigeon louse fly (Pseudolynchia canariensis) has been associated with transmission of Haemoproteus spp., but also pigeon adenovirus 1 and possibly pigeon pox virus.
Mosquitoes
They have been shown to cause irritation and to act as a vector for avian malaria in penguins, as well as causing the spread of other blood parasites and viruses, such as West Nile fever eastern and western encephalitis viruses.
Gnats
Gnats can transmit blood-borne parasites such as Leucocytozoan spp.
Ticks
These can rapidly be fatal to birds, possibly due to the presence of a toxin in the saliva of the tick. They are seen relatively uncommon, but can occur in raptors being used for hunting, as ticks are ground dwellers. They are also common in aviary-kept birds which are overhung by surrounding trees. The ticks may be carried on wild birds and so drop-off roosts and nests into an aviary beneath. Again, they may transmit many bacteria, such as members of the Rickettsia family, as well as blood-borne parasites and viruses.
Endoparasites
The parasite Giardia spp. can infest cockatiels leading to internal irritation, which causes the affected bird to pluck the feathers over its flanks and ventrum. In addition, severe ascarid nematode infections have been associated with feather plucking in psittacine birds.
Viral diseases affecting the skin
Psittacine beak and feather disease
Psittacine beak and feather disease (PBFD) is caused by a member of the circovirus family. There is evidence of the presence of different strains of the virus in different species. As its name suggests, it is mainly seen in Psittaciformes, although many other orders of birds have been found to be affected. Some species may recover from infection, such as many lorikeets and lories. In many species, circoviral disease is a terminal infection. Grey parrots (Psittacus erithacus) are an example where two main forms of the disease are commonly seen: An acute form which is seen in juvenile birds that present with clinical anaemia and immunosuppression (and frequently aspergillosis); and a chronic form which causes increasing feather, nail and beak dystrophia, death finally occurring due to dysphagia and inanition (see Figure 13.2). In many birds, the first feathers to be affected are the powder feathers – this is more obvious in cockatoos which normally have a very dusty beak – those infected with circovirus have a shiny beak free of dust.
Figure 13.2 PBFD in an African grey parrot – note the overlong beak, which is splitting, and some poor feather quality.

In Eclectus parrots and Lovebirds, early circoviral disease can present with self-mutilation making circoviral infection a differential in feather plucking. In Budgerigars, the disease is known as ‘French moult’, with birds from 4–5 weeks becoming depressed then showing necrosis of developing feathers. Some may die after 1–2 weeks with diarrhoea and crop stasis. Transmission of circovirus in psittacine birds is by feather dander, oral and faecal routes. In Columbiformes, a typical disease pattern occurs in young squabs between 2 months and 1 year of age. Incubation periods are 2 weeks. Clinical signs include anorexia, diarrhoea, weight loss, respiratory disease and lethargy. Death commonly occurs within 3–5 days. The disease produces immunosuppression and therefore concurrent infections are common-most, typically Chlamydophila psittaci or Aspergillus spp. infections.
Diagnosis is based on polymerase chain reaction (PCR) demonstration of the viral antigen in blood or feather pulp – it is advised that both are submitted as frequently one can be negative depending on the stage of the disease. Others have suggested liver biopsy in Grey parrots as this appears to be the target organ (Grund et al., 2005). Alternatively, histopathology of skin biopsies can demonstrate viral inclusions and at postmortem in young birds, depletion of B lymphocytes from the Bursa of Fabricius with viral inclusions in the bursa, thymus and in the bone marrow. There is no treatment for the virus although avian gamma interferon therapy has been tried with varying degrees of success. Vaccination has been attempted, but if the bird is already infected, then vaccination has been shown to actually deteriorate the bird’s condition.
Polyomavirus
Avian polyomavirus (APV) infection is common and causes systemic disease in various species of psittacine, gallinaceous, passerine and raptor birds. In budgerigars (Melopsittacus undulatus), it is the cause of budgerigar fledgling disease/feather duster disease. In this condition, neonates in infected flocks may die suddenly at around 10–15 days. Others may develop abdominal distension, reduced feathering and haemorrhages under the skin. Some may show neurological signs such as ataxia and tremors of the head. If infected after 15 days of age, they will often survive but develop feather abnormalities preventing flying. In juvenile finches (primarily Gouldian finches), clinical signs include weight loss, fluffed appearance, diarrhoea and dehydration. A polyomavirus has also been identified in canaries (Serinus canaria) with similar signs plus loss of feathers and some neurological signs. Many of these had secondary infections.
Diagnosis is based on clinical signs and PCR testing of cloacal swabs or tissues at postmortem. Testing indicates that the canary and finch strain are different (Shivaprasad et al., 2009). Clinical gross postmortem and basophilic intranuclear inclusion bodies on histopathology are suggestive.
There is no treatment for this condition, although there is now a vaccine in the United States (Biomune II Psittimune®). Detecting virus DNA particles from a cloacal swab or faecal sample is the standard test.
Avipox viruses
These can occur in all avian species, although none have so far been recorded in Strigiformes. Signs include pox-like lesions, occurring predominantly on the face, eyelids, feet and cere. These become secondarily infected, and may lead to more extensive lesions. Respiratory tract disease is also seen and may cause sloughing of the lining of the trachea and smaller airways, causing asphyxiation. Transmission may occur directly from bird to bird, and by flying insect vectors. The pox virus affecting canaries is particularly virulent and may cause serious mortality in canary flocks and has been associated with lung neoplasia.
Diagnosis is based on demonstrating the classical Bollinger bodies, seen due to virus particles inside infected cells, as well as the clinical signs described.
Papillomavirus
This may lead to proliferative skin masses on the feet and legs of passerine birds and they are considered enzootic in some populations of waterfowl, Gruiformes and Ciconiiformes and may mimic mite infestations. Rarely in psittacine birds, proliferative lesions on the face and around the beak may be seen. Papillomas of the digestive tract are now thought not to be caused by a papillomavirus but rather by a herpesvirus. Diagnosis is by histopathological demonstration of intranuclear inclusion bodies. There is no treatment at present.
Bacterial disease affecting the skin
Examples include Salmonella typhimurium var. copenhagen joint infection of pigeons, which may then erupt as a boil visible on wing joints such as the elbow; and Staphylococcal spp. and E. coli infections of the feet of many species, which cause bumblefoot.
Bumblefoot
In raptors, it is more frequently seen in falcons. Persistent pressure on the same parts of the sole of the foot when perching restricts the blood flow to these areas. This will ultimately lead to hypoxia and death of tissues, followed by open sores and deep pedal infections. Bacteria most commonly seen such as E. coli and Staphylococci spp., but yeasts such as Candida spp. may also be seen in cases that have been on antibiotics for prolonged periods of time. Many categorisations for the differing stages of bumblefoot have been described, the most basic being proposed by Cooper (1985):
- Type I: This is when the injuries are restricted to smoothing of the normally papillated underside of the foot. There may or may not be the presence of a corn and sometimes a mild scab.
- Type II: This is the next stage, wherein the mild scabs become deeply infected. This condition may also be caused by the raptor puncturing its own foot with one of its claws. The bacteria involved include Staphylococcus aureus, E. coli, Pseudomonas spp. and occasionally yeasts.
- Type III: This is the worst lesion, with deeper structures such as bones within the toes, ligaments etc., all becoming infected. These birds are often not possible to treat. The feet are very swollen and painful and hot to the touch.
More recently, bumblefoot has been further refined in classification into five categories from the mildest at I, with early devitalisation of the plantar aspect of the foot, to severe osteomyelitis of the phalangeal bones at V (Oaks, 1993; Remple, 1993).
Fungal diseases affecting the skin
These tend to be secondary to other injuries, or skin mutilation. Candida albicans has been reported in lorikeets and associated with hypovitaminosis A. It has also been seen in lovebirds with feather loss around the eyes, beak and neck with scaling.
Malessezia spp. have been associated with pruritus in galahs, eclectus parrots, mynah birds and cockatiels (Reavill et al., 1990).
Dermatophytosis has been recorded in Galliformes where it is known as favus, and is generally seen most commonly around the wattles and comb.
Allergic skin conditions
There is some evidence that allergic skin disease is a problem in Psittaciformes. Serological tests do not seem to be reliable in birds, but intradermal skin testing has been shown to be useful in determining allergens and has been shown to give significantly different results in feather plucking and normal birds (MacWhirter et al., 1999). Codeine phosphate is used as the positive control as it gives more consistent results than histamine (Colombini et al., 2001). The intradermal skin test is carried out using the skin either side of the keel over the pectoral muscles which limits the amount of allergens which can be tested for in one go and also due to the thin nature of the avian skin requires the bird to be anaesthetised with isoflurane. However, the results indicate that some feather plucking parrots do have an allergic response to allergens, such as sunflower seeds, house dust mites and Aspergillus spp. Hypovaccination has not been tried on any major scale but this author has had some effects with hypovaccines.
Miscellaneous diseases affecting the skin
Split keel
Split keel is commonly seen in young, overweight, hand-reared birds, particularly those which have been carelessly wing clipped. It occurs when the bird attempts to fly off a high perch, and, either due to poor wing muscling or lack of flight feathers, drops like a stone and hits the ground with force. This often splits the thin skin covering the prominent keel area and this split may become secondarily infected. This delays healing, and may necessitate the use of antimicrobials or even surgery to achieve wound healing.
Ulcerative skin disease
Ulcerative skin disease can be due to bacterial infections, for example, Staphylococcus spp., or may be due to fungal infections such as infection by Aspergillus spp. Birds most commonly affected are species such as lovebirds, cockatoos and cockatiels.
The bird traumatises the skin underneath each wing, leading to a weeping ulcerative dermatitis. The infections may be primary or secondary. Little is known about the true initial causes of this condition, although some suggestions recently have included allergic skin conditions similar to those seen in dogs.
Psittacosis/chlamydophilosis
Systemic infection with Chlamydophila psittaci has been implicated in some parrots with feather plucking and generally poor feather quality (Figure 13.3).
Figure 13.3 Infection with Chlamydophila psittaci may cause many conditions, one of which may be feather plucking as seen here.

Changes in feather colouration and structure
This may occur due to a lack of one nutrient, such as the paler colour seen in some canary breeds, for example, red factors, when deprived of vitamin A. Vitamin A in particular is required for the production of the yellow and red colouration of feathers. Other colour changes which can be seen include the following:
- Vasa parrots will develop white feathers rather than their normal grey, when they are infected with PBFD virus.
- Red colouration of the grey feathers in African grey parrots may also be seen with hepatic disease and with PBFD virus.
- Black feathers appearing in green-coloured birds such as Amazons can suggest liver disease.
Fret marks
Fret marks are another common feather abnormality seen in birds with a history of illness. These are breaks in the integrity of the feathers due to poor production of the interlocking barbs. This produces a noticeable band on all of those feathers which were growing at this time. The presence of fret marks suggests some systemic disease or malnutrition at the time the feathers were being produced, and can be a useful external indicator of a bird’s recent past health, although they say nothing about its current health status.
Hormonal skin disease
Hypothyroidism has been recorded in chickens and African grey parrots. Clinical signs include slow growth and poorly coloured feathers, which often lack the interlocking barbules and so appear ragged. Diagnosis is based on the thyroid stimulating hormone (TSH) test where 1 IU/kg of TSH is administered intramuscularly after measurement of basal T4 levels. T4 levels are then measured 24 hours later, over which time a 2.5 times increase in T4 should have occurred. Failure to do so suggests hypothyrodism. It has been suggested that an underlying cause of hypothyroidism may be a dietary deficiency of iodine.
Tumours and feather cysts
The more common skin tumours include subcutaneous lipomas, and the so-called xanthoma seen particularly along the distal wings of birds (e.g. budgerigars). This is more an accumulation of fat deposits within the cells, producing a thickening of the area, and a characteristic yellow colouring of the skin.
Feather cysts are seen commonly in budgerigars and canaries (Figure 13.4). The condition occurs when a new growing feather fails to find its way through the skin surface, and so continues to grow while trapped under the skin. The feather cyst becomes an enlarging caseous nodule, which can ulcerate and become secondarily infected. It is thought that the condition is hereditary and the affected birds should be removed from breeding stock.
Oil spills
Sea birds and shore birds alike become coated with crude oil, making them flightless and removing their waterproofing. Many also succumb to the toxic and irritant effects of the oil itself.
Treatment is based on a number of principles which include stabilisation of the often dehydrated and malnourished bird, followed by prevention of further irritation and absorption of oil across mucous membranes, and finally removal of the oil from the pelage (feathers and skin) (Chapter 6).
Heavy metal toxicosis
Acute heavy metal (principally lead and zinc) toxicosis has been associated with neurological and renal disease in birds. However, chronic lead toxicosis in particular is also implicated in feather plucking. Older houses often have wooden surfaces that have decades of lead based paints, which parrots are adept at chewing off! Kitchen units use lead in the ‘leading’ of glass doors. Solder and many pewter items contain lead. Diagnosis is via blood testing – the cut-off level for lead is >0.2 ppm (20 ug/dL or 1.25 umol/dL) measured in lithium heparin whole blood. Levels >0.5 ppm (50 ug/dL or 2.6 umol/dL) are diagnostic. Some argue that as lead is not required for any bodily system function and therefore the presence of any lead is significant.
Wing tip oedema
This, as its name suggests, is seen clinically as swelling and oedema of the wing tips (carpus distally) and is most commonly associated with frostbite in raptors. It will often lead to avascular necrosis of the wing tips once the oedema has subsided if not treated quickly (see Figure 13.5).
Digestive disease
Crop
Ingluvitis
Ingluvitis is inflammation of the crop. It can be caused by an overgrowth of the yeast Candida albicans or bacteria such as E. coli and other Gram-negatives. Candida albicans infection tends to be confined to the crop and gastrointestinal tract. It is often a sequel to prolonged antibiotic therapy such as tetracycline treatment of chlamydophilosis or in neonate psittacine birds. Clinical signs usually involve regurgitation and delayed crop emptying. Diagnosis is by visualising the thickened crop lining, which has been likened to a towel surface and by demonstration of typical peanut-shaped budding yeasts. Presence of pseudohyphae indicates deeper tissue involvement and worsens the prognosis.
Ingluvitis can also be due to parasites such as Trichomonas spp., which are a common cause of regurgitation of seed in budgerigars and cockatiels. In pigeons and raptors, Trichomonas spp. cause a condition, known as ‘canker’ or ‘frounce’, which results in caseous yellow nodules at the corners of the oropharynx and the proximal oesophagus. It can however spread throughout the body and result in high levels of mortality, particularly in raptors. The feeding of wild-caught pigeons to raptors is a prime means of transmitting this particular pathogen. If a raptor owner is to feed wild-caught pigeon, then it should first be frozen for 3–4 weeks and then thoroughly defrosted before being fed, so as to kill off any Trichomonas spp. present. Diagnosis of the causal agent is by crop wash. The volume of crop wash saline used varies from 0.5 ml in a budgerigar up to 10 ml in a large macaw. The sample should then be examined using both Gram’s stain and Diff-Quik® style dichrome stains. Trichomonas spp. may be difficult to pick up using this method, as it is often in the lining of the crop. Treatment has to be given on the assumption that this disease exists, once all of the other possibilities have been ruled out. Willette et al. (2009) describe trichomoniasis as the most clinically significant parasitic disease in birds of prey. Certain species however seem more; for example, resilient-peregrine falcons, which naturally feed on wild Columbiformes, are less likely to suffer from trichomoniasis. Conversely, Northern Goshawks (Accipter gentilis), gyrfalcons (Falco rusticolus) and barn owls (Tyto alba) are much more susceptible. In addition, young birds seem more susceptible.
Bacterial infections causing ingluvitis due to E. coli, Pseudomonas spp. and Aeromonas spp. are also not uncommon, particularly in juvenile birds and neonates.
Sour crop
Sour crop in Psittaciformes is associated with juvenile birds. It occurs when the contents of the crop ferment, producing a foul-smelling acidic environment. This condition may be life-threatening. In raptors, it may be associated with overconsumption of food. The crop has a neutral pH and so if food is present for too long a period of time it can ferment and go off leading to sour crop.
Crop impaction
Crop impaction occurs in juvenile birds that may overeat. Some may occur due to poor motility associated with neurological conditions such as lead poisoning or proventricular dilatation disease. Alternatively, they may eat the floor covering, and, if this is composed of shavings or sawdust, impaction may occur (this is particularly common in pheasants and other young game birds).
Capillariasis
Capillariasis is the name given to the infestation of the crop lining with the nematode Capillaria spp. This is mainly a problem for pigeons and raptors, but is also seen in Passeriformes (e.g. finches and canaries), as well as species of Galliformes (e.g. pheasants and grouse). It is much rarer in Psittaciformes.
Clinically, raptors flick their head from side to side when eating, and will regurgitate. In pigeons, acute infections are associated with high mortality because of intense vomiting. In cases of capillariasis, characteristic bipolar eggs may be seen in the faeces. The life cycle is often indirect and may involve earthworms.
The parasite Streptocara spp. have been associated with oesophageal damage in waterfowl similar to that caused by Capillaria spp. in other birds.
Crop burns
These are frequently seen in hand-reared Psittaciformes. They occur when the owner has fed the juvenile bird a rearing formula porridge that has been microwaved and not thoroughly stirred and allowed to stand resulting in ‘hot spots’ within the mixture, which will cause local burns. These can be full thickness, causing the skin to slough after 7–10 days.
Foreign bodies
Many larger Psittaciformes may consume foreign objects, which become lodged in the crop and may cause irritation, retching, and may lacerate the thin crop lining. Retrieval of the object via endoscopic examination under anaesthesia is advised, with repair of any lacerations.
Associated crop disorders
The lack of iodine in an all seed diet will cause thyroid gland enlargement (goitre). This enlarged gland will press on the crop and proximal oesophagus, so limiting its ability to empty and fill. Often seen in budgerigars, they will adopt a horizontal posture lying across the perch with the head slightly raised. There is frequent intermittent regurgitation of seed.
Proventriculus
Proventriculitis
Inflammation of the true stomach (proventriculus) can be caused by the yeasts Candida spp. and Macrorhabdus ornithogaster (formerly known as ‘Megabacteria’). Signs can include the vomiting of food, the passing of undigested seed in the faeces, and in the case of Psittaciformes, anorexia, diarrhoea and weight loss.
In budgerigars, the incidence of Macrorhabdus ornithogaster infection is relatively high. Many birds are carriers with no clinical signs, but a percentage of a flock will show a ‘going light’ syndrome. They will eat voraciously, but lose weight, often passing undigested seed in their faeces. Diagnosis is by finding the typical bacillus-like Gram-positive chains of the yeast in faeces.
‘Megabacteriosis’ is seen in a wide host of other species including finches, canaries, parrotlets, cockatiels, lovebirds, ostriches and domestic poultry (ducks, chickens, turkeys and geese).
Proventricular dilatation disease
Proventricular dilatation disease (PDD) has been reported in over 50 species of psittacine and nonpsittacine birds. It was first reported in Macaws (aka ‘Macaw wasting disease’) and its current name is derived from the lymphoplasmacytic ganglioneuritis, which damages the nerves supplying the proventriculus resulting in progressive muscular flaccidity leading to a maldigestion syndrome, starvation and eventually death (see Figures 13.6 and 13.7). The disease appears to be able to affect the central nervous system as well as the myenteric and so affected birds may show signs of ataxia, nervous tremors, central blindness, fitting and torticollis (Steinmetz et al., 2008). The median age of onset appears to be 3–4 years but has been reported as early as 5 weeks of age and as old as 17 years (Phalen, 2006a; Smith, 2009).
Figure 13.6 Severe emaciation and death is the clinical outcome of proventricular dilatation syndrome (breast feathers have been manually removed to show extent of weight loss).
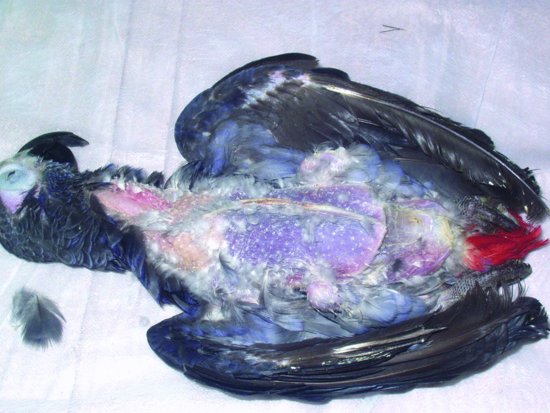
Figure 13.7 An enlarged proventriculus (cream structure curving around the darker liver) is seen on examination.
The virus is an avian Bornavirus (Gancz et al., 2009; Gray et al., 2009; Shivaprasad et al., 2009) with Koch’s postulates being fulfilled in the cockatiel (Nymphicus hollandicus) (Hoppes et al., 2010) and Patagonian Conures (Gray et al., 2010). They cause most of the clinical signs by stimulating the body’s own immune system. Multi-bird households have a significantly higher incidence of infection with avian Bornavirus than single bird households (71.4% versus 51.3%). Infection may be associated with higher incidences of feather plucking (76% of positive cases showed plucking behaviour) (Zantop, 2010). Transmission is suspected to be faeco-oral but respiratory transmission is also suspected (Perpinan et al., 2007). Incubation period may be rapid as witnessed by the young age of some cases, but may also take up to 7 years to manifest itself.
Definitive diagnosis is difficult. Clinically, the disease has been diagnosed based on histopathology of a proventricular or crop biopsy. Diagnosis by crop biopsy is less accurate ([66–76%] Doolen, 1994; Gregory et al., 1996); although trying to ensure a blood vessel is incorporated to increase the likelihood of sectioning a nerve increases the chances of success. Proventricular biopsy although more accurate is more challenging due to the risk of postoperative coelomitis. Other supportive diagnostic tests include positive contrast radiography demonstrating a dilated proventriculus and displacement of the ventriculus to the right side with prolonged emptying times. These signs may be mimicked by other disease such as neoplasia, foreign bodies and heavy metal poisoning. PCR serology and crop biopsy have so far showed a poor correlation between the three (Clubb & De Kloet, 2010). Villaneuva et al. (2010) have shown that western-blotting techniques to test for antibodies to avian Bornavirus was effective; however, of the 117 psittacine birds detected as positive, only 30 could be confirmed on histopathology suggesting that subclinical carriers of avian Bornavirus are common. The same study also showed that many seronegative birds also were positive on detected antigen in faeces by PCR again showing further discrepancies.
Proventricular/ventricular impaction
If insoluble grit is fed, the grit builds up can block the gizzard. Other causes of impaction are seen in juvenile cockatoos (Cacatua spp.) and African grey parrots (Psittacus erithacus), which often compulsively ingest items in their environment such as fragments of wood, plastic etc. These birds present as vaguely unwell, losing condition, rarely with vomiting although recurrent bacterial enteritis has been reported (Speer, 1998).
Endoparasites
Helminths
Ascaridia spp: Ascarids are commonly seen in cage and aviary birds, particularly those that have a deep litter or earth floor to their aviary, as this allows maturation of any worm eggs that are passed in the faeces. Species seen include Ascaridia platycerci (psittacine birds), A. hermaphrodita (in a Hyacinth macaw), A. columbae (found in psittacine birds and Columbiformes), A. columbae (Columbiformes) and A. galli (found in Galliformes and psittacine birds).
Infected individuals may show no external signs, but if parasitised heavily enough they may lose weight, have diarrhoea or, in severe cases, may experience intestinal blockage and death. Ascarids have a direct life cycle. This means that the eggs passed in the faeces of an infected bird, after a few days, can be directly infectious to another bird without having to be taken up by an intermediate host. Diagnosis is made by finding the thick-walled eggs in the faeces of the affected bird.
Capillaria spp
In pigeons, Capillaria obsignata burrows into the lining of the intestine where it can cause severe diarrhoea and regurgitation, often with fatal consequences. Diagnosis is by demonstrating the presence of the bioperculate eggs in the faeces or regurgitated material produced by the infected bird. Its life cycle is direct, as with the ascarid family.
Other digestive system worms seen in birds
Other species of worm found in the digestive system are largely nematodes and include Ornithostrongylus quadriradiatus which is found in the intestine of pigeons. It is a blood-sucking worm, similar to the hookworms. Its eggs are thin walled and more spherical than the Capillaria spp. eggs.
Waterfowl
There are many different species of nematode worms. Echinuria spp. cause a high mortality rate in young ducks and swans. It is transmitted via the intermediate host Daphnia spp., the water flea. It can produce tumour-like lumps in the lining of the proventriculus and gizzard, which may lead to partial blockage of these organs.
Epomidostomum and Amidostomum spp. which infest the gizzard of geese, cause bleeding into the lumen, diarrhoea, enteritis and weight loss. In young birds, it causes growth retardation and even death.
Game birds
Heterakis isolonche is a significant cause of mortality in young pheasants, causing severe damage to the caecal lining. In many Galliformes, such as turkeys and chickens, this worm carries another pathogen with it – Histomonas meleagriditis, a single-celled protozoan parasite that can cause significant focal liver necrosis, giving the disease its name of black spot.
Corvids and thrushes
Porrocaecum spp. have been associated with weight loss and intestinal/stomach granulomas.
Non-helminth digestive system parasites
Motile protozoan parasites:
Giardia psittaci are commonly found in cockatiels and budgerigars. Signs of the disease vary, and in the case of the cockatiel, it has been associated with a deficiency in vitamin E, producing feather plucking.
Diagnosis is by finding the parasites on faecal smears. The sample is suspended in saline and examined using the x400 microscope lens. The sample must be fresh, as the parasite disintegrates rapidly once the faeces dry. When fresh, movement of the organism which has eight flagellae can be observed.
Hexamita spp. are commonly found in pigeons. It produces weight loss and diarrhoea. It is seen in young birds towards the end of the breeding season when environmental contamination is high. Diagnosis is by finding large numbers of the motile, eight-flagellae-bearing, elongated protozoa using the x400 microscope lens on a slide of fresh faeces suspended in saline.
Trichomonas spp. may affect the intestines as well as the crop.
Cochlosoma spp. are a flagellate single-celled protozoal parasite, which has been associated with widespread mortalities in Australian finches. It causes diarrhoea, dehydration and moulting abnormalities particularly in birds between 10 days and 6 weeks of age.
Coccidiosis
Eimeria spp. infest many species, from pigeons and cage birds to Galliformes and waterfowl. They contribute to the ‘going light’ syndrome where the bird loses condition. In pigeons, heavy burdens of E. columbarum and E. labbeana may affect racing performance. Clinical signs may appear before oocysts are detected in the faeces. In canaries, Isospora canaria affects canaries of 2 months of age and older producing diarrhoea and emaciation, primarily damaging the duodenum which may be oedematous at postmortem. In hill mynah birds, Eimeria spp. have been associated with haemorrhagic enteritis.
Caryospora spp. cause crop and intestinal damage. There is often high mortality in young birds, with adults exhibiting abdominal discomfort. There are over seven species of Caryospora known to affect birds of prey, the two most commonly seen in captivity being C. falconis and C. neofalconis (Forbes and Simpson, 1997; Heidenreich, 1997). Transmission is by direct means although there is evidence that rodent prey can act as a heteroxenous host. Clinical signs include general debility, diarrhoea, weight loss and anorexia and are most commonly seen in young raptors between 3 and 6 months of age. The parasite sexually reproduces in the gut, but encysted forms can be found in the muscle and central nervous system, and result in an encephalitis with neurological signs such as torticollis. Merlins (Falco columbarius) appear particularly susceptible with sudden death occurring. Diagnosis can be made on clinical signs and the presence of huge numbers of oocysts in the faeces with a typical single sporocyst with eight sporozoites within it.
In canaries and other Passeriformes, Atoxoplasma spp. may be found. It spreads from the gut into the mononuclear white blood cells and is carried through the bloodstream affecting organs such as the liver, resulting in hepatomegaly known as ‘black spot’ because it is visible through the thin skin of the canary’s abdomen. Diarrhoea or death may be seen, but the disease may be asymptomatic.
Diagnosis of coccidiosis is by finding the oval to round oocysts in the bird’s faeces using sugar flotation methods, or by direct smear of the liver.
Cryptosporidium spp:
This coccidian organism appears to be able to infect any epithelial surface and so has been reported in the gastrointestinal, respiratory and urinary tracts. It has a direct life cycle and the transmission route is faeco-oral, in food (other avian prey) or by respiratory aerosol. In psittacine birds, the proventricular form is the most commonly observed, with birds co-infected with Macrorhabdus ornithigaster being most likely to die (Messenger & Garner, 2010).
Bacterial gastrointestinal disease
Bacteria such as E. coli, Campylobacter spp., Clostridium spp., Pseudomonas spp., Salmonella spp., Yersinia spp. and Chlamydophila psittaci may all cause diarrhoea and even toxaemia, septicaemia and death. Salmonella spp. may be particularly difficult to treat as the bacteria become entrenched in the lining of the intestines. The main salmonellae seen in birds are Salmonella typhimurium and S. arizonae. Clinical signs resemble those seen in yersiniosis but are generally more chronic. However, significant mortality can occur, particularly in finches and other small passerine birds. In pigeons, S. typhimurium var. Copenhagen typically produces swollen joints, especially on the wings referred to as ‘boils’ by pigeon racers and fanciers. In addition, weakness, lethargy, green urates, diarrhoea and death of young hatchlings may all point to a salmonella problem. In pigeons, after paramyxovirus 1, salmonellosis is the next most common cause of neurological signs and may also mimic mycobacterial disease when infecting bones. Culture and sensitivity testing using specific Salmonella spp. culture medium should always be performed on avian faecal samples in cases of diarrhoea and weight loss. Escherichia coli are generally absent from the intestines of passerine and psittacine birds (Dorrestein, 2009), but are typically found in 97% of all pigeon intestinal tracts (Harlin & Wade, 2009). Clinical signs of E. coli infection include diarrhoea and sudden death, and may be associated with epizootic mortalities in imported finches. Clostridium spp. have been associated with gastrointestinal disease and death of a wide range of birds. They may be found in the gastrointestinal tract of many species notably raptors. Clostridium perfringens is considered a normal part of the flora of a raptors digestive system. However, as with clostridial bacteria in rabbits, overgrowth may occur in poor conditioned birds resulting in enterotoxaemia. In addition, food spoiled by clostridial toxins will result in rapid death of the raptor.
In addition, the mycobacterium Mycobacterium avium, the cause of avian tuberculosis, is often seen in waterfowl and raptors. It inhabits the gut and associated organs and is thus spread via the faecal–oral route. It is often implicated in chronic wasting diseases, and produces classical caseous nodules throughout the gut. Diagnosis is made on Ziehl–Neilsen stains demonstrating the acid-fast bacteria, and bacterial culture of the faeces. It is not treatable and, as it is a zoonotic disease, affected birds should be euthanased.
Viral gastrointestinal disease
Duck plague virus
The duck plague virus is a member of the alpha herpesvirus family. It affects both ducks and geese. It is spread in the faeces and oral secretions and may be carried latently with no clinical disease, or can cause death with no premonitory signs. Alternatively, it may cause violent diarrhoea, anorexia and neurological tremors. It can be associated with mass outbreaks in ducks. Death usually occurs within 3–12 days of infection, and 1–10 days after developing clinical signs. Diagnosis is made on the clinical signs and isolation of the virus from faeces, or on demonstration of intranuclear inclusion bodies in the gut wall on postmortem.
Avian papillomatosis
This condition is thought to be due to a herpesvirus. It occurs in members of the Psittaciformes family causing papillomas throughout the digestive system frequently in the cloaca. In the cloaca, it often leads to irritation and eversion or even prolapse due to repeated straining. Other clinical signs include regurgitation, recurrent bouts of enteritis, passage of blood in the faeces and infertility. Papillomatosis is also linked with a high incidence of liver and pancreatic cancer in affected birds.
Diagnosis is made in the live bird by visualising the papillomas aided by applying 5% acetic acid to the lesion, which will cause the papillomas to turn white while normal mucosal folds will remain pink.
Liver disease
Haemochromatosis
Haemochromatosis is where excessive amounts of iron are deposited in the liver. Birds affected are commonly members of the toucan, toucanette, mynah and starling families. In the wild, these species often live in iron poor soil areas, and their digestive systems are therefore adapted to absorb as much iron as possible. When presented with an iron-rich diet, high levels of vitamin C (which encourages iron absorption from the gut), too much iron is absorbed. This iron is then deposited in the liver, leading to damage and failure.
Clinical signs include ascites, dullness, dyspnoea (due to the ascites pressing on the air sacs), abdominal swelling and sudden death. Diagnosis is based on species, clinical signs, and liver biopsy to assess iron levels. This can be a dangerous procedure, particularly if the bird has ascites. There is then a real danger of rupturing air sacs and drowning the bird.
Avian tuberculosis (Mycobacterium avium infection)
Avian tuberculosis may produce liver disease, causing classical caseous nodules throughout the structure, and is also associated with intestinal disease.
Other liver bacterial diseases
Any bacteria, such as Salmonella spp., E. coli and Pseudomonas spp., which breaches the gut wall may affect the liver directly via the enterohepatic circulation. In addition, the pansystemic infection of Chlamydophila psittaci will cause hepatitis manifested, as with so many hepatic disorders, by green-to-yellow coloured urates. Diagnosis is based on clinical signs, biochemistry and isolation of the bacteria from the gastrointestinal tract and, if possible, from hepatic swabs.
Viral liver diseases
Psittacid herpesvirus isolates 1, 2 and 3 have been recorded, all of which can cause ‘Pacheco’s disease’ in psittacine birds. Old World psittacine birds appear more resistant to infection than New World ones with Amazon parrots and conures being considered the most susceptible. Clinical signs may be sudden death for New World birds, or a more prolonged course of depression, anorexia, regurgitation and green diarrhoea before death in more resistant species. Occasionally, haemorrhagic diarrhoea may be seen. Some cockatoos have been reported as surviving infection (although probably remain persistently infected). Transmission appears to be in oropharyngeal secretions and faeces. At postmortem, the liver is often bronze in colour and enlarged. The kidneys may also be enlarged and the intestines and brain congested. Intranuclear inclusion bodies (Cowdry type A) with hepatocellular necrosis are suggestive. Diagnosis can also be made using PCR technology on cloacal or oropharyngeal swabs. A vaccine is available in the United States.
Raptor herpesviruses
Currently have three distinct isolates: Falconid herpesvirus 1 (FHV1); strigid herpesvirus 1 (SHV1) and accipitrid herpesvirus 1 (AHV1). All cause hepatitis and splenitis with typical herpesvirus inclusion bodies. Wernery and Kinne (2004) suggest that transmission is via the ocular/intranasal route in falcons.
Falconid herpes virus
This virus affects Falconidae and occurs mainly in Europe and the Middle East. It is usually fatal, particularly in gyrfalcons and causes liver and spleen swelling and necrosis, bone marrow necrosis and gut inflammation with haemorrhage. It is transmitted directly from bird to bird and via prey items.
Strigid (Owl) herpes virus
This virus causes liver, spleen and bone marrow necrosis and is specific to the owl family. It is invariably fatal within 3–9 days. Yellow nodules may be found in the gut and the pharyngeal mucosa. It is spread in urine and faeces, as well as respiratory secretions. Some birds that survive will remain latently infected. There is no treatment or vaccine available.
Pigeon herpesvirus
This can cause a mild pharyngitis/oesophagitis with cellular necrosis in young birds. Older birds are frequently immune but remain persistent carriers. Anorexia, regurgitation and sometimes neurological signs in conjunction with green urates are suggestive. Inclusion body hepatitis can be seen on biopsy of affected livers.
Duck viral hepatitis
Duck viral hepatitis affects chiefly young ducklings under 3 weeks of age and is caused by a member of the Picornaviridae. It is rapidly fatal and produces severe liver damage and haemorrhage from the liver surface.
Avian reovirus
Not all are pathogenic but some can cause significant disease particularly in budgerigars and Grey parrots. High mortalities have been reported in the United Kingdom in budgerigar flocks (Manvell et al., 2004; Pennycott, 2004). Clinical signs include anorexia, fluffed up appearance, dyspnoea, nasal discharge, diarrhoea and sudden death. Postmortem reveals hepatitis, nephritis, serositis with ascites, pneumonia and splenitis with subcutaneous haemorrhages. There is often lymphoplasmacytic infiltrates of various organs, haemosiderosis, haemorrhage and fibrin deposition. Infections are commonly associated with other pathogens such as C. psittaci, adenovirus and M. ornithogaster infections.
Avian leukosis/sarcoma virus
This family group of viruses is known to induce a tissue borne leukaemia in Psittaciformes, Galliformes and Passeriformes (such as the canary). It destroys the liver and kidneys, which become infiltrated by rapidly dividing lymphocytes. Hen birds seem more susceptible than cock birds, and it is shed in the faeces, oral/respiratory secretions and semen. It is also passed in genetic material from mother to young in the egg. Clinical signs include hepato- and renalomegaly, ascites, respiratory distress, weight loss, green coloured urates, polydipsia and polyuria. There is no treatment.
Hepatic lipidosis
Hepatic lipidosis is usually diet related. High fat diets (such as the all-seed diets so beloved of Psittaciformes) and lack of exercise lead to obesity and fat deposition in the liver cells or hepatocytes. Liver function suffers as a consequence. This condition may also be seen in raptors that are not working, but are still being well fed. Affected birds are often sleek, plump birds, which then become dull and lethargic, and may exhibit signs varying from ascites, to respiratory distress and clotting defects.
Toxic liver conditions
Toxins from a number of sources may cause hepatic damage in the avian patient. These include the heavy metal toxicoses such as lead and zinc poisoning. It may also include other metals such as copper, chromium and mercury.
Lead poisoning
Lead poisoning is frequently seen in waterfowl, which have inadvertently eaten lead shot from hunting or fishing. Psittaciformes may also be affected, as they will consume or destroy many household items that contain lead. These include
- Lead window effects on kitchen units
- Lead weights in curtains or blind drawer cords
- Lead in the foil used for wine bottles
- Metallic cloths used for sanding/polishing metals
- Lead paints in older houses
Clinical signs include
- Weakness (S-shaped neck of the swan)
- Lethargy, vomiting, passage of blood in the faeces (very common in Psittaciformes)
- Passage of lime green faeces (very common in raptors)
- Chronic non-regenerative anaemia
- Seizures
- Kidney and liver damage
- Death
Diagnosis is by finding blood lead levels in excess of 0.2 ppm (12.5 mmol/L). Those in excess of 0.4–0.6 ppm (25–37.5 mmol/L) are diagnostic. In addition, radiography will often show lead particles as radiodense areas in the proventriculus and ventriculus.
Zinc poisoning
It is mainly seen in Psittaciformes shortly after they have been moved into a new cage (‘new wire cage disease’). Occasionally, the finish of these cages is of a poor quality and a fine powder of zinc oxide forms the surface of the wires. The parrot manoeuvres itself around the cage with feet and beak, and takes in small volumes of this powder on a daily basis. After 4–6 weeks, liver and kidney damage will occur, and the bird may present weak, lethargic, having seizures and with anaemia. Other sources of zinc include some forms of coin, some plastics and other alloys. Diagnosis is made on clinical signs, history and finding zinc blood levels above 2 ppm (2000 mg/L).
Other poisons affecting the liver
Aflatoxins released by Aspergillus spp., are known to be hepatotoxic and carcinogenic, as are the organic poisons found in rapeseed, ragwort and the castor bean. Treatment is rarely possible.
Pancreatic disease
Diabetes mellitus is a common condition in budgerigars and cockatiels. It may be hereditary, although pancreatitis can result in the development of this disease.
Clinical signs include polydipsia and polyuria, often with dramatic weight loss despite a healthy appetite.
Diagnosis is made on the clinical signs in conjunction with persistently raised blood glucose (often above 25 mmol/L up to 44 mmol/L). Some glucose may be found in the urine normally, but high levels (>1%) are strongly suggestive of disease.
Pancreatitis can be seen in birds. It has been linked with infections due to Paramyxovirus III (e.g. cockatiels). The passage of partially digested seed or pasty tan coloured faeces may be seen combined with weight loss.
Respiratory disease
Upper respiratory tract
Nostrils
Clinically often feathers are stained just above the nares, and there may be sneezing and head flicking due to the discharge.
Rhinoliths, concretions of dried secretions that form a ball of solid material blocking the entrance to the nasal passages, may be caused by dietary insufficiencies such as hypovitaminosis A, and/or may involve local or deeper infections due to bacteria such as Mycoplasma spp. (e.g., pigeons), or Chlamydophila psittaci (e.g. Psittaciformes).
Fungi, such as Aspergillus spp., or viruses, such as the avipox virus group, are also the causes. The mite Knemidocoptes spp. may also contribute to the blockage of the nostrils (e.g. Psittaciformes and raptors).
Some species of parrot, such as Amazons, are susceptible to nasal irritation in dry environments, and in the presence of feather dander, produced by African grey parrots and cockatoos.
A congenital defect is the absence of a patent internal choanal slit. This prevents the normal nasal secretions from draining ventrally into the oropharynx. The secretions then present as a clear nasal discharge.
Theromyzon tessulatum is the duck nasal leech and is a common parasite. It may lead to secondary infections of the nasal passages.
Pigeon herpesvirus (PHV) affects young (<6 months) pigeons. It causes a nasal discharge, sneezing and necrotic plaques to form inside the mouth and upper airways. It may also cause the cere to become discoloured and crusty. In severe cases death may occur. There is no specific treatment or vaccine available, although many will survive.
Sinusitis may be seen in conjunction with rhinoliths and nasal discharges. It may be visible as a swelling on the face, often ventral to the eye in the area of the infraorbital sinus.
Lower respiratory tract
Trachea
Gape worm infection: Syngamus trachea is found in Galliformes, pigeons and raptors (e.g. buzzards). This parasite uses the earthworm as its intermediate host before finally maturing in the windpipe of the afflicted bird. There it causes irritation and secondary infection, and may be present in sufficient numbers to suffocate the bird. Diagnosis is made by finding the characteristic Y-shaped worms (the male and female worms are permanently joined in copulation!) in the mucus of the mouth and trachea.
Avipox virus
In Amazon parrots, a severe form of avipox virus may cause the sloughing of the lining of the windpipe, resulting in dyspnoea, pneumonia and even death from secondary infection. All avipox viruses cause pox lesions on the face, particularly around the eyes and mouth, the pharynx and trachea, but may also produce lesions on the feet. Secondary infections are common. In pigeons there are vaccines available. Diagnosis is confirmed by finding the characteristic Bollinger bodies that are the reproductive centres of virus replication within the host cells.
Aspergillosis
The fungus Aspergillus spp., can produce a growth that blocks the windpipe in a matter of days (see Figure 13.8). This condition is particularly common in raptors and many Psittaciformes such as African grey parrots. Diagnosis is discussed below.
Figure 13.8 An aspergilloma growing in the trachea of this raptor resulted in asphyxiation and its death.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree