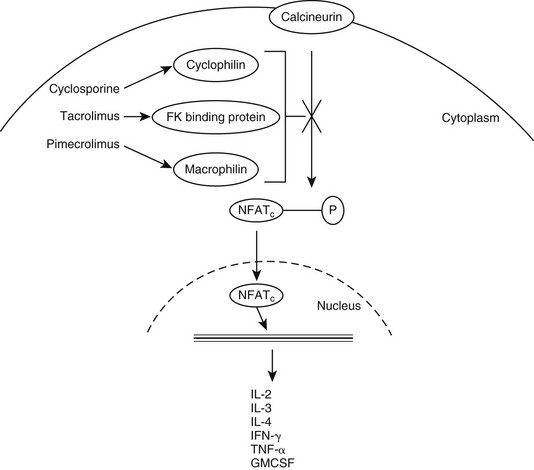
Web Chapter 45 Calcineurin inhibitors, including drugs such as cyclosporine, tacrolimus, and pimecrolimus, are an important class of immunomodulators that have been at the forefront of this genre in the last few years (see Chapter 59). Calcineurin is a key enzyme in the activation of T lymphocytes. It functions in the induction of gene transcription for a number of inflammatory mediators, including many interleukins (e.g., interleukin-2, interleukin-3, interleukin-4), granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-α, and interferon-γ (IFN-γ). Calcineurin inhibitors function by binding to a carrier protein with a high affinity for calcineurin, preventing its activity (Web Figure 45-1). They also have been shown to inhibit the activation of mast cells, basophils, eosinophils, keratinocytes, and Langerhans cells. Both cyclosporine and tacrolimus decrease the number and activity of epidermal dendritic cells and down-regulate the expression of the high-affinity immunoglobulin E receptor (FcεRI) on Langerhans cells. Calcineurin inhibitors have been used in humans and animals for many years. Specifically, the use of oral cyclosporine for treatment of atopic dermatitis (AD) has received much interest recently in veterinary medicine, whereas topical calcineurin inhibitors have been a hot topic in human dermatology literature. Web Figure 45-1 Mechanism of action of calcineurin inhibitors. GMCSF, Granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; NFATc, nuclear factor of activated T cells; P, phosphorylation; TNF, tumor necrosis factor. Oral cyclosporine has been well studied for its role in managing AD and a number of other dermatologic diseases (see Chapter 92). It is a highly lipophilic cyclic polypeptide with a molecular weight of 1202 kD. Because of its molecular size and structural and biologic differences from the other calcineurin inhibitors, its use as a topical treatment for inflammatory skin disease has been unrewarding and limited to only anecdotal reports of success. Marsella and Nicklin (2002) performed early studies on the topical use of tacrolimus for treatment of AD in dogs. In the initial pilot study, which used a 0.3% lotion formulated from the oral product, investigator scores for erythema were lower (indicating less erythema) in tacrolimus-treated dogs than in placebo-treated dogs, but owner scores for pruritus were not significantly different for the two groups. However, a subsequent randomized, double-blind, placebo-controlled crossover study using commercially available 0.1% tacrolimus ointment (Protopic) found more encouraging results (Marsella et al, 2004). In this study, treatment with topical tacrolimus was evaluated in 12 dogs that were diagnosed with AD. After 4 weeks of once-daily application, investigator scores improved significantly in the tacrolimus-treated group, whereas no significant differences between pretreatment and posttreatment signs were detected in the placebo group. When the dogs were divided based on severity of disease, those with localized disease were found to show significantly greater improvement than those with generalized disease according to both owner and investigator assessments. In conclusion, this study found that tacrolimus ointment decreased clinical signs of AD over a 4-week period with minimal adverse effects or safety concerns. A third study investigated the efficacy of 0.1% tacrolimus ointment in the treatment of localized lesions of AD on the front paws of dogs (Bensignor and Olivry, 2005). All dogs had lesions at other sites, but these were not included in either treatment or evaluation. At the end of the 6-week study the primary investigator found that all dogs that completed the study showed a more than 50% decrease in lesional scores for the evaluated areas (based on grading of erythema, lichenification, oozing, and excoriations). This study did not evaluate pruritus. A recent study evaluated the tolerability and safety of a 0.1% compounded tacrolimus solution (in olive oil) applied to the external ear canals of atopic dogs with normal otoscopic findings, including intact tympanic membranes (Kelley et al, 2010). In this prospective, double-blind, placebo-controlled trial a colony of high IgE–producing beagles validated as a model for AD were studied. This tacrolimus solution was well tolerated by these dogs, with no adverse topical reactions noted and no evidence of hearing loss. However, the olive oil vehicle may have predisposed to an increase in Malassezia overgrowth and mild otitis. These results, along with favorable outcomes reported with the use of an ear wick containing 0.1% tacrolimus ointment to treat chronic noninfectious otitis in human patients, suggests that further investigation of this therapy is warranted (Harth et al, 2007). An open-label study was performed by my practice evaluating 0.1% topical tacrolimus in the management of immune-mediated diseases such as discoid lupus erythematosus (DLE) and pemphigus erythematosus (PE). In this study 10 dogs with DLE and 2 dogs with PE were treated with a commercially available 0.1% tacrolimus ointment (Griffies et al, 2004). All dogs had lesions localized to the nasal planum and adjacent regions of the muzzle. Owners applied 0.1% tacrolimus ointment to the affected areas every 12 hours. Each dog’s lesions were assessed for degree of erythema, crust, ulceration or erosion, depigmentation, and scarring and were evaluated 2, 4, and 8 weeks after the start of therapy. Ten of 12 dogs exhibited improvement in clinical lesions associated with PE or DLE (5 with excellent response, 5 with partial response) over the 8-week trial. Of the dogs that showed progress, 8 of 10 were treated with tacrolimus alone by the end of the study and remained in remission at 12 weeks. Two of these dogs followed for an additional 2 years continued to do well with topical tacrolimus alone.
Topical Immunomodulators
Calcineurin Inhibitors
Cyclosporine
Tacrolimus
Canine Atopic Dermatitis
Immune-Mediated Diseases
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Chapter 45: Topical Immunomodulators
Only gold members can continue reading. Log In or Register to continue
