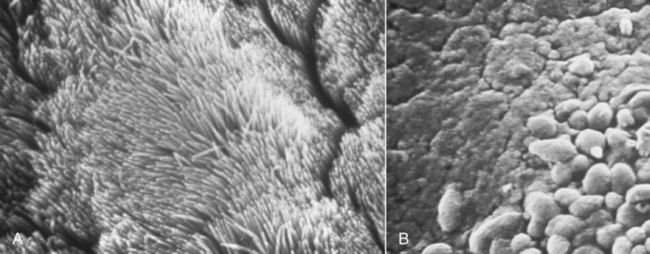
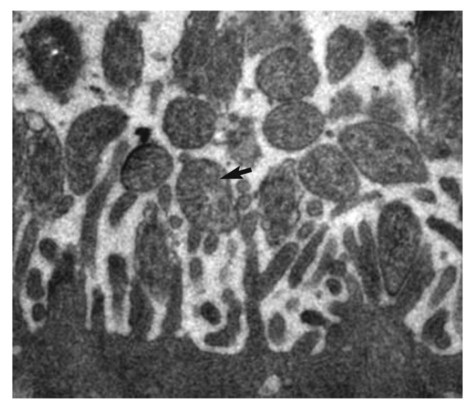
Canine infectious respiratory disease (CIRD), also known by the common names including canine infectious tracheobronchitis, “kennel cough,” “canine cough,” “canine croup,” acute contagious respiratory disease, and canine contagious respiratory disease complex. CIRD describes any contagious, acute-onset respiratory infection of dogs, typically involving the upper respiratory tract. The etiology of infection is complex and involves several viral and bacterial pathogens acting alone or synergistically.15 Table 6-1 and Web Table 6-1 summarize the various known and potential pathogens associated with CIRD. Although any one of the individual pathogens described can cause clinical signs consistent with CIRD, co-infection involving two or possibly more pathogens simultaneously colonizing the upper respiratory tract of the same patient can occur. Kennel-housed dogs may be particularly susceptible to co-infection. Two new viruses, canine influenza virus (CIV, see Chapter 23) and canine respiratory coronavirus (CRCoV), have emerged since 2000, both of which are known to infect the respiratory tract of dogs. Only CIV has been shown to cause clinical signs of CIRD. Whereas Bordetella bronchiseptica and canine parainfluenza virus (CPIV) have commonly been described as the principal pathogens recovered from dogs involved in CIRD outbreaks, CIV and CRCoV each pose reasonable risk of infection in susceptible dogs, especially those housed within high-density environments. The risk of CIRD continues to expand as the list of pathogens increases and the consequences of comorbidity, which are not well studied, become reality. The potential for co-infection is high, which makes diagnosis, treatment, and prevention even more problematic in the clinical setting. Although other viruses and bacteria have been recovered from dogs with CIRD, their role in the pathogenesis of infection is less clear. TABLE 6-1 Organisms Associated with Canine Infectious Respiratory Disease WEB TABLE 6-1 Pathogens Associated with Canine Infectious Respiratory Disease IN, intranasal. bSee text and Table 6-4. Refer to Web Appendix 3, Canine and Feline Biologics Manufacturers and Products Available Worldwide. CPIV is a single-stranded RNA virus belonging to the family Paramyxoviridae and is closely related to simian virus 5 (see Chapter 7).7 Of the various viruses known to be associated with CIRD, CPIV has perhaps received the most attention as a principal respiratory pathogen. CPIV is found in dogs throughout the world and is known to cause acute-onset, short-lived, but highly transmissible cough; kennel-housed dogs are at greatest risk of exposure. Although natural infection may result in detectable serum antibody for periods of up to 3 years, immunologic protection is generally determined by IgA concentrations at the level of the respiratory mucosa. A positive serum antibody titer to CPIV does not necessarily correlate well with protection from clinical disease.108,109 Several other viruses have been implicated in CIRD. Canine adenovirus (CAV) type 2 (CAV-2), a DNA virus of the family Adenoviridae (see Chapter 4), can cause acute infectious laryngotracheitis in dogs that is characterized by acute-onset cough; clinical signs may be inapparent to mild in uncomplicated infections. Unlike CAV-1, where noncanid wildlife can serve as environmental reservoirs, the host range of CAV-2 beyond canids is uncertain. Dogs that are not immunized against either CAV-1 (cause of infectious canine hepatitis) or CAV-2 are particularly susceptible to infection. CIV is a dog-adapted strain that originated from genetic alterations in the H3N8 virus circulating in horses (see Chapter 23). In 2003, CIV was isolated from several kennel-housed greyhounds in Florida experiencing an outbreak of acute upper respiratory signs, especially nasal discharge and cough.27 With outbreaks having been identified in at least 30 states and the District of Columbia in the United States, CIV is justifiably included as a primary pathogen of CIRD. Coronaviruses (CoV) are now classified into at least three groups.45 A possible fourth group is under consideration and includes the cause of severe acute respiratory syndrome (SARS) in humans: SARS-CoV. The three major types of CoVs have been isolated from dogs and are classified as either group I or II CoVs. In 2003, a group II CRCoV, closely related to but genetically distinct from bovine CoV, was isolated from dogs housed in a rehoming center in the United Kingdom.44,47,47 This virus should be differentiated from the group of type I enteric canine coronaviruses (CCoV; see Chapter 8). CRCoV was subsequently isolated from the upper respiratory tract of household dogs in Japan.93,131 Seroepidemiologic and genetic evidence of CRCoV infection in dogs has been reported in Italy, Japan, Korea, Canada, the United States, New Zealand, and Greece.* One retrospective study suggests the likelihood that CRCoV has been present in dogs in North American since 1996.42 A seasonal occurrence has been suggested, with infection risk being highest in the winter months rather than summer. CRCoV is more likely to be recovered from the respiratory tracts of dogs over 1 year of age.101 Although the CRCoV is considered to be highly contagious and can be isolated from naturally infected dogs with CIRD, dogs with uncomplicated infections appear to develop either subclinical or mild disease. Clinical disease has not been substantiated by experimental inoculation of virus into susceptible dogs. Similarly, the original bovine CoV, from which CRCoV was thought to originate, causes only subclinical infection of pups.74 In natural situations, co-infections or host factors may be responsible for the ability of CRCoV to produce clinical illness; however, further studies will be needed to document this suspicion. Until natural or experimental challenge can be shown to cause respiratory signs in susceptible dogs, CRCoV should not be considered a primary pathogen of CIRD. A single outbreak of a highly virulent strain of CCoV belonging to group II was documented in seven pups housed in a pet shop in Italy.15 Affected dogs developed severe gastrointestinal, neurologic, and respiratory signs; deaths were reported within 2 days of the onset of signs. Type I and type II CoVs were found in the intestinal content; however, only the type II virus was present in many other organs, including the lungs, in association with lesions. This clinical disease was very similar to that caused by the group IV SARS-CoV infection of humans. At this time, the virulent pantropic CCoV is not considered to be a constituent pathogen of the CIRD complex. Canine distemper virus (CDV) infection can cause acute-onset cough, and nasal and ocular discharge and is occasionally included among the various pathogens involved in CIRD. However, CDV infection is not characteristically limited to the respiratory tract and more frequently causes pneumonia with bacterial complications (see Chapter 3). Generalized canine distemper typically progresses rapidly, involving multiple organ systems, particularly the central nervous system, skin, eyes, and bone. Although CDV can act synergistically with CPIV and B. bronchiseptica and may ultimately lead to significant respiratory disease and death, it is not regarded as a primary pathogen in the etiology of the generally self-limiting syndrome defined as CIRD (also see Chapter 3). Canine herpesvirus (CHV) is most commonly implicated as a serious systemic, frequently fatal, infection of puppies under 2 weeks of age (see Chapter 5). In adult dogs, infection is often subclinical or latent. However, acute or reactivated CHV may cause respiratory signs of rhinitis and pharyngitis. CHV was identified in ocular swabs in a higher percentage of dogs with conjunctivitis as compared to those without.84 Evidence of CHV infection has been identified in lung and tracheal samples collected from dogs housed in a rehoming center.47 Serologic studies involving kenneled and household dogs suggest relatively high prevalence.44 However, recrudescence of latent CHV may lead to intermittent viral excretion after physiological (co-infection) or pharmacologic (glucocorticoid) stress. Therefore, the role of CHV in CIRD has been controversial. However, a nosocomial outbreak of CIRD, attributed solely to CHV infection, occurred in a referral veterinary hospital where most of the affected dogs had been receiving a variety of immunosuppressive treatments including glucocorticoids, chemotherapy, surgery or irradiation.74b Pneumovirus, closely related to murine pneumovirus was cultured from nasal and pharyngeal specimens of dogs experiencing acute upper respiratory disease in a shelter.107a Other viruses, predominantly CPIV and CIV were also isolated. Co-infections or immunocompromised host issues might lead to potential clinical illness caused by this virus. However, pneumovirus antibodies have been found in both clinically healthy and ill kennel dogs suggesting widespread exposure and questionable pathogenicity (see Web Appendix 5). The spectrum of potentially pathogenic bacteria found in the upper respiratory tracts of dogs adds to the complexity of the pathogenesis and clinical outcome of CIRD. However, it is unlikely that any of the several bacterial species implicated for their role in canine respiratory disease carries more importance than B. bronchiseptica. Nine species of Bordetella have been identified. Three are known for their ability to cause respiratory disease. Unlike the human host-restricted Bordetella pertussis, the cause of whooping cough, and Bordetella parapertussis, B. bronchiseptica is infectious for a wide range of mammals, including humans. Over the past decade, research findings have provided insight into the complexity of the virulence patterns unique to the Bordetellae and explain how these bacteria are able to exist both as a commensal organism within the upper respiratory tract and as a highly virulent pathogen (see Pathogenesis). These findings support the role of B. bronchiseptica as a critical cofactor in the pathogenesis of CIRD. Other bacteria recovered from the respiratory tracts of dogs with CIRD include Streptococcus spp., Pasteurella spp., Pseudomonas, and various coliforms.104,119 Their role as primary pathogens versus secondary invaders is less clear. One exception may be Streptococcus equi subsp. zooepidemicus, which has been identified as a primary cause of an acute-onset, contagious, and often fatal bronchopneumonia in dogs housed in shelters and research kennels (see Chapter 33).21 Its also has been considered as a copathogen in some of the fatal CIV outbreaks in greyhounds (see Chapter 23). There are limited reports describing the role of mycoplasmas as primary respiratory pathogens in dogs. Mycoplasmas are fastidious, prokaryotic microbes that are distinguished from bacteria by the fact that they are enclosed in a cytoplasmic membrane but lack a distinct cell wall.11,80,80 Nonhemotropic mycoplasmas, acholeplasmas, and ureaplasmas have been recovered from the nasopharyngeal and laryngeal mucosae of clinically healthy dogs and cats. Mycoplasma spp. have also been isolated from the lower respiratory tracts of dogs (especially Mycoplasma cynos) and cats (Mycoplasma felis) with pneumonia.11,105,105 There are questions as to the precise role these organisms play in CIRD. See Chapters 32 and 87 for further discussion of this controversy. CIRD is among the most common causes of acute-onset respiratory diseases of dogs. Despite the implementation of vaccination programs against the principal pathogens associated with CIRD, outbreaks continue to be reported throughout the world. Immunity derived from vaccination does not confer complete protection against infection, clinical disease, or organism shedding. Although many agents have been associated, CIRD usually results from infection by any one of four principal pathogens (B. bronchiseptica, CPIV, CIV, CAV-2). Simultaneous infection with any two or more of these pathogens in the same patient is likely to pose a significant risk for increased morbidity among susceptible dogs. Dogs housed in private and commercial kennels, pet shops, animal shelters, and boarding facilities (including veterinary hospitals) are at significantly greater risk of exposure and infection compared to household pets.8,11,40,56,104 Furthermore, the risk of syndemic infection, which is two or more pathogens synergistically infecting dogs within a population, is probably greatest among shelter-housed dogs. Despite the diversity of pathogens associated with CIRD, most viruses and B. bronchiseptica are efficiently transmitted in the same manner to naïve dogs from infected dogs through oronasal contact with aerosolized respiratory secretions. Among dogs housed in high-density populations, transmission most likely occurs after direct contact with infected dogs or contact with aerosolized microdroplets, either from infected dogs or through freshly contaminated dishware, human hands, and other fomites. For the principal viruses involved, the onset of signs and duration of viral shedding are similar. Clinical signs (usually initially cough) can develop within 1 to 3 days after exposure. Viral shedding begins within a few days after infection, whereas the duration of viral shedding usually ranges from 6 to 10 days, after which the virus load diminishes substantially. On the other hand, B. bronchiseptica, an obligate extracellular bacterium, and the mycoplasmas are able to elude immune recognition and destruction for weeks or months.7,8 Therefore, high numbers of bacteria are likely to be expelled in respiratory secretions of healthy-appearing dogs for extended periods. With respect to environmental survival, B. bronchiseptica can be transmitted from dogs to cats. B. bronchiseptica has been shown to survive in lake water, without added nutrients, for up to 24 weeks and replicate in natural waters for at least 3 weeks at 37° C.99 CPIV is among the most common causes of highly contagious acute-onset cough in dogs throughout the world. Classified in the family Paramyxoviridae, the virus is a single-stranded RNA containing seven genes that encode eight proteins. Infection risk correlates with a high population density of dogs. Experimental challenge has shown that CPIV replicates primarily in the epithelium of the nasal mucosa, pharynx, larynx, trachea, and bronchi. There, its cytolytic replication causes denuding of the respiratory epithelium (Fig. 6-1). Viremia is uncommon, although CPIV has been recovered from spleen, liver, and kidneys in dogs with mixed infections. Viral shedding persists only 8 to 10 days after infection, during which time aerosolized respiratory secretions can transmit virus to susceptible dogs. CPIV infection is characterized by self-limiting cough, often characterized as a high-pitch “honking” cough likely attributable to vocal fold swelling. Laryngitis and tracheitis may be associated with episodic gagging and expectoration. A serous nasal discharge, tonsillitis, with or without pharyngitis, may develop. In the absence of complicating secondary infections, clinical signs resolve spontaneously within 6 to 14 days of onset. However, co-infection with B. bronchiseptica or another respiratory virus, especially in puppies, is likely to culminate in an extended, potentially severe clinical course. CIV causes a respiratory illness in experimentally infected dogs and is associated with fever and viral replication and seroconversion.27 Clinical signs range from inapparent to mild respiratory signs (cough) lasting 2 to 3 weeks; some naturally infected dogs develop serious, life-threatening lower respiratory disease. Respiratory airway and regional lymph node inflammation is apparent in most experimentally infected dogs; however, a few dogs develop pulmonary lesions typical of pneumonic consolidation.35,36 Viral replication and shedding are of relatively low level, most productive in the first 4 days after infection, and generally does not last for more than 10 days. Shedding was observed in subclinically affected dogs as well. Rapid spread of infection occurs after entry of the virus into a group of housed dogs. See Chapter 23 for a further review of the pathogenesis of this infection. CAV-2 and CAV-1 are both known to infect respiratory epithelium and cause respiratory signs, especially cough. CAV-2, however, is the predominant adenovirus recovered from dogs with CIRD and is recognized worldwide among dogs that have been vaccinated against either CAV-1 or CAV-2. Despite vaccination of domestic dogs, wild canids may serve as reservoirs for canine exposure. After oronasal transmission, the virus replicates in surface epithelium of the nasal cavity, pharynx, tonsillar crypts, and the goblet cells in the trachea. CAV-2 is not limited to the upper respiratory tract, because it may also infect nonciliated cells in the bronchi and type 2 alveolar epithelium. Virus has also been recovered from bronchial and retropharyngeal lymph nodes. Virus replication peaks at 3 to 6 days postinfection then declines commensurate with the increase of local antibody. By 9 days postinfection, virus cannot be isolated. The most dramatic respiratory lesions associated with CAV-2 infection centers on the distal airways and lung. Bronchitis and interstitial pneumonia are reported in dogs experimentally challenged with CAV-2, although the clinical illness caused by the infection is inapparent or minor. Mortality associated with severe pneumonia has been observed in natural infection of pups of 4 weeks of age or younger.1 Clinical manifestations associated with CAV-2 infection are worsened when bacterial or viral co-infection occurs.12,26,26 CAV-2 has been identified in a higher rate of dogs with naturally acquired conjunctivitis as compared to clinically healthy control dogs.84 See Chapter 4 for further information on the pathogenesis of canine adenovirus infections. CDV infection can result in respiratory signs that are indistinguishable from other causes of CIRD, particularly during the early states of infection. However, the respiratory tract is not the primary target of CDV. Infection in highly susceptible dogs progresses systemically, resulting in death. Although CDV can be considered a significant copathogen in the pathogenesis of CIRD, it is not regarded as a primary agent. The pathogenesis of CDV is described in detail in Chapter 3. CRCoV seropositive dogs have been identified in several countries, and viral isolation has been confirmed.2,74,87,131 However, seropositivity in dogs has not consistently been associated with clinical signs of respiratory disease. Neither experimental nor natural CRCoV challenge studies have been published. There is currently no direct evidence that CRCoV is a primary pathogen in CIRD. It is likely that CRCoV infection, like CPIV and CAV-2, follows direct contact with infectious respiratory secretions. After a short incubation period, clinical signs, if present, are characteristic of CIRD and include cough, nasal discharge and occasionally inappetence. In one clinical study involving shelter dogs with signs of respiratory disease, CRCoV was most commonly recovered from dogs with mild cough.48 In naturally infected dogs, virus was frequently identified in the nasal cavity, tonsils, and trachea, and less likely to be recovered from the lung and bronchial lymph nodes. Studies of the etiology of CIRD in which dogs with CRCoV were identified also found dogs to be co-infected with other respiratory pathogens, particularly B. bronchiseptica and CPIV.45 CHV infection is characteristically described as a fatal, systemic infection in puppies under 2 weeks of age and has been associated with fetal death. However, experimental infection of dogs with herpesvirus has been reported to cause rhinitis and pharyngitis as well as signs consistent with CIRD. Although the virus has been isolated from dogs with upper respiratory signs, the role of CHV in CIRD remains unclear. See Chapter 5 for further information on the pathogenesis of this disease. Mammalian reoviruses are capable of infecting virtually all mammals, including humans. All three serotypes, 1, 2, and 3, have been isolated from dogs with respiratory disease or enteritis. Although it is isolated from the upper respiratory tracts of dogs, the role of canine reovirus as a principal pathogen in CIRD is uncertain. Reovirus challenge studies conducted in healthy dogs have been inconclusive. It has been suggested that CRV acts synergistically with other pathogens to cause signs consistent with CIRD.16 B. bronchiseptica, a gram-negative, aerobic coccobacillus, was recognized as a primary cause of respiratory disease in dogs in the early 1970s. Today, it is regarded as one of the principal causative agents of CIRD and may be a critical complicating factor in dogs simultaneously infected with a viral pathogen. B. bronchiseptica has regularly been isolated from the upper respiratory tracts of clinically healthy dogs and cats and those with signs of respiratory disease, particularly cough and nasal discharge. The complex pathogenesis of the bordetellae helps explain how clinical manifestations of infection can range from mild upper respiratory signs in otherwise healthy dogs to serious disease characterized by pneumonia and death. Of particular importance, yet poorly studied, are the consequences of comorbidity involving any of the pathogens known to be associated with CIRD. In a study of community-acquired pneumonia of dogs, dogs with pneumonia attributed to B. bronchiseptica were more likely to have been obtained from a pet store and have more prolonged hospitalization than dogs with other incriminated bacteria.104 The consequences of B. bronchiseptica infection are variable and difficult to predict in the clinical setting. The complexity of this bacteria-host interaction can be attributed to a virulence control system, nearly identical to that found in B. pertussis and B. parapertussis, encoded by the bvgAS locus. BvgA and BvgS (Bordetella virulence genes A and S, respectively) are key constituents of the two-component transduction system that regulate the proteins responsible for the expression of virulence factors. BvgAS controls at least three distinct phenotypic phases of B. bronchiseptica, Bvg+, Bvgi, and Bvg−, which are believed to be involved with colonization, transmission, and survival (persistence), respectively. Interestingly, expression of a particular phase appears to be under the influence of changing environmental conditions during the course of infection. The relevant signals that influence and regulate the bvgAS locus have not yet been determined. Given the ability of B. bronchiseptica to regulate its virulence and the likelihood that co-infected dogs tend to experience more serious clinical disease than dogs infected with single agents, viral co-infection (e.g., CPIV or CIV) conceivably could influence B. bronchiseptica virulence and the clinical manifestation CIRD in an individual dog. B. bronchiseptica virulence factors and their function are summarized in Web Table 6-2. WEB TABLE 6-2 Principal Virulence Factors for Bordetella Bronchiseptica and Summary of Actions From Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Microbiol Rev 18:326–382. Studies have provided insight into the pathogenesis of B. bronchiseptica and how this unique pathogen is able to reside in the respiratory tracts of healthy dogs (and cats), then, under the influence of yet undefined stimuli, recognize specific receptors on ciliated respiratory epithelium, effectively attach to and colonize these cells, elude immune destruction, and subsequently cause tissue injury.* Bacterial attachment to ciliated respiratory epithelium represents a critical first stage of B. bronchiseptica infection (Fig. 6-2). Under the control of the BvgAS two-component system, filamentous fimbriae are believed to mediate the binding of Bordetella to the respiratory epithelium17 and are actually required for persistence in the trachea. Another adhesin, filamentous hemagglutinin, is the dominant attachment factor for Bordetella and may have a role in overcoming the clearance activity of the mucociliary apparatus. Early in the course of infection, and before epithelial cell injury or mucous production, B. bronchiseptica induces ciliostasis, a critical event in the pathogenesis of infection in that it not only prevents bacterial clearance but also enhances further colonization.3 After colonization, B. bronchiseptica uses several complex intrinsic mechanisms to express a series of exotoxins and endotoxins, also called virulence factors or determinants. These virulence factors not only lead to direct cellular injury but also impair immune recognition and clearance.6,40,56,103,132
Canine Infectious Respiratory Disease
Etiology
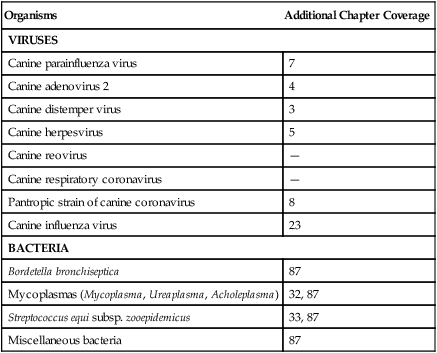
Organisms
Additional Chapter Coverage
VIRUSES
Canine parainfluenza virus
7
Canine adenovirus 2
4
Canine distemper virus
3
Canine herpesvirus
5
Canine reovirus
—
Canine respiratory coronavirus
—
Pantropic strain of canine coronavirus
8
Canine influenza virus
23
BACTERIA
Bordetella bronchiseptica
87
Mycoplasmas (Mycoplasma, Ureaplasma, Acholeplasma)
32, 87
Streptococcus equi subsp. zooepidemicus
33, 87
Miscellaneous bacteria
87
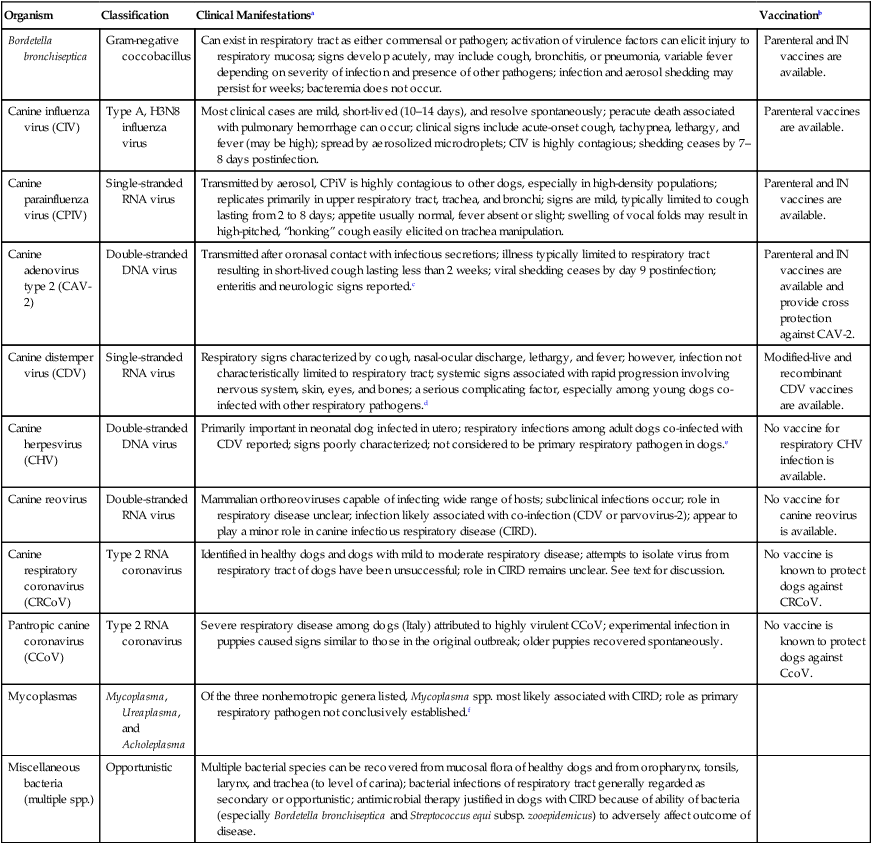
Organism
Classification
Clinical Manifestationsa
Vaccinationb
Bordetella bronchiseptica
Gram-negative coccobacillus
Can exist in respiratory tract as either commensal or pathogen; activation of virulence factors can elicit injury to respiratory mucosa; signs develop acutely, may include cough, bronchitis, or pneumonia, variable fever depending on severity of infection and presence of other pathogens; infection and aerosol shedding may persist for weeks; bacteremia does not occur.
Parenteral and IN vaccines are available.
Canine influenza virus (CIV)
Type A, H3N8 influenza virus
Most clinical cases are mild, short-lived (10–14 days), and resolve spontaneously; peracute death associated with pulmonary hemorrhage can occur; clinical signs include acute-onset cough, tachypnea, lethargy, and fever (may be high); spread by aerosolized microdroplets; CIV is highly contagious; shedding ceases by 7–8 days postinfection.
Parenteral vaccines are available.
Canine parainfluenza virus (CPIV)
Single-stranded RNA virus
Transmitted by aerosol, CPiV is highly contagious to other dogs, especially in high-density populations; replicates primarily in upper respiratory tract, trachea, and bronchi; signs are mild, typically limited to cough lasting from 2 to 8 days; appetite usually normal, fever absent or slight; swelling of vocal folds may result in high-pitched, “honking” cough easily elicited on trachea manipulation.
Parenteral and IN vaccines are available.
Canine adenovirus type 2 (CAV-2)
Double-stranded DNA virus
Transmitted after oronasal contact with infectious secretions; illness typically limited to respiratory tract resulting in short-lived cough lasting less than 2 weeks; viral shedding ceases by day 9 postinfection; enteritis and neurologic signs reported.c
Parenteral and IN vaccines are available and provide cross protection against CAV-2.
Canine distemper virus (CDV)
Single-stranded RNA virus
Respiratory signs characterized by cough, nasal-ocular discharge, lethargy, and fever; however, infection not characteristically limited to respiratory tract; systemic signs associated with rapid progression involving nervous system, skin, eyes, and bones; a serious complicating factor, especially among young dogs co-infected with other respiratory pathogens.d
Modified-live and recombinant CDV vaccines are available.
Canine herpesvirus (CHV)
Double-stranded DNA virus
Primarily important in neonatal dog infected in utero; respiratory infections among adult dogs co-infected with CDV reported; signs poorly characterized; not considered to be primary respiratory pathogen in dogs.e
No vaccine for respiratory CHV infection is available.
Canine reovirus
Double-stranded RNA virus
Mammalian orthoreoviruses capable of infecting wide range of hosts; subclinical infections occur; role in respiratory disease unclear; infection likely associated with co-infection (CDV or parvovirus-2); appear to play a minor role in canine infectious respiratory disease (CIRD).
No vaccine for canine reovirus is available.
Canine respiratory coronavirus (CRCoV)
Type 2 RNA coronavirus
Identified in healthy dogs and dogs with mild to moderate respiratory disease; attempts to isolate virus from respiratory tract of dogs have been unsuccessful; role in CIRD remains unclear. See text for discussion.
No vaccine is known to protect dogs against CRCoV.
Pantropic canine coronavirus (CCoV)
Type 2 RNA coronavirus
Severe respiratory disease among dogs (Italy) attributed to highly virulent CCoV; experimental infection in puppies caused signs similar to those in the original outbreak; older puppies recovered spontaneously.
No vaccine is known to protect dogs against CcoV.
Mycoplasmas
Mycoplasma, Ureaplasma, and Acholeplasma
Of the three nonhemotropic genera listed, Mycoplasma spp. most likely associated with CIRD; role as primary respiratory pathogen not conclusively established.f
Miscellaneous bacteria (multiple spp.)
Opportunistic
Multiple bacterial species can be recovered from mucosal flora of healthy dogs and from oropharynx, tonsils, larynx, and trachea (to level of carina); bacterial infections of respiratory tract generally regarded as secondary or opportunistic; antimicrobial therapy justified in dogs with CIRD because of ability of bacteria (especially Bordetella bronchiseptica and Streptococcus equi subsp. zooepidemicus) to adversely affect outcome of disease.
Viruses
Bacteria and Mycoplasmas
Epidemiology
Pathogenesis
Viruses
Canine Parainfluenza Virus
Canine Influenza Virus
Canine Adenovirus Type 2
Canine Distemper Virus
Canine Respiratory Coronavirus
Canine Herpesvirus
Canine Reovirus
Bacteria and Mycoplasmas
Virulence Determinant
Action
Filamentous hemagglutinin
Surface-associated and secreted protein; may be required for attachment to ciliated epithelium and colonization; highly immunogenic; may also have a role in B. bronchiseptica–mediated immunomodulation.
Fimbriae
Filamentous cell surface structure required for persistent tracheal colonization (ciliated epithelium).
Pertactin
Surface protein; enhances protective immunity.
Vag8
Outer membrane protein.
Adenylate cyclase
Calmodulin-activated toxin with dual adenylate cyclase/hemolysis activity; acts as an anti-inflammatory and antiphagocytic factor during infection.
Type III secretion system
Allows Bordetella to translocate effector proteins into host cells; required for persistent tracheal colonization; inhibits host immune response; causes cell death.
Dermonecrotic toxin
Heat-labile toxin; induces necrosis in vitro.
Tracheal cytotoxin
Causes mitochondrial bloating, disruption of tight junctions, and damage to ciliated cells.
Lipopolysaccharide (LPS)
Large molecule found in outer cell membrane of gram-negative bacteria; acts as an endotoxin; O antigen of B. bronchiseptica, a constituent of LPS, can prevent action of antibody against bacteria.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Canine Infectious Respiratory Disease