Chapter 24 Blood Transfusion and Blood Substitutes
The use of both blood transfusions and fluid therapy must be carefully assessed before inclusion in a patient’s treatment plan, and the veterinarian should evaluate the risk/benefit ratio for each patient. Volume overload, electrolyte disturbances, and transmission of infection can occur from administration of pathogen-contaminated blood products or fluids.31,66,130 Despite the potential negative effects of transfusion, most veterinarians view it as lifesaving therapy, allowing the transfusion recipient to receive other necessary treatments such as surgery, chemotherapy, or medical care.56
Three major differences exist between the more commonly used fluids and blood products. The differences between crystalloid or colloid solutions and blood products are their immunogenicity, availability, and cost. The immunogenicity of blood products stems from the proteins and cellular material in the blood. Because crystalloid solutions lack proteins and cellular material, they are not considered immunogenic; however, certain colloid solutions such as hydroxyethyl starch have been reported to cause acute anaphylaxis in rare instances in humans.96 The mechanism of this reaction is unknown.
Limited availability differentiates blood products from crystalloid and colloid solutions. Crystalloid and colloid solutions are readily available because they can be manufactured according to market demand. Only a living animal can produce blood, and the donor’s physiologic capability limits production. The small number of commercial canine and feline blood banks providing a convenient source of blood for the veterinary practitioner further limits availability of blood for transfusion (Box 24-1). Furthermore, blood products require a more regulated storage environment and have a significantly shorter shelf life than crystalloid or colloid solutions, making blood a less convenient product to store and use in a veterinary hospital.
Box 24-1 Veterinary Blood Banks
Buddies for Life (Michigan veterinary hospitals only)
Animal Blood Resources International (formerly Midwest Animal Blood Services and Animal Blood Bank)
http://www.vet.upenn.edu/RyanHospital/SpecialtyCareServices/BloodBank/tabid/432/Default.aspx
http://vetbloodbank.com/index.html
Nearly 20 years ago, veterinarians estimated costs associated with transfusions, but an exact analysis of cost is lacking. In 1992, the estimated cost of a 500-mL whole blood transfusion ranged from $25 to more than $300.56 The cost of 500 mL of lactated Ringer’s solution is about $1.
Despite the fact that the first documented transfusion was given to a dog in 1665 by Richard Lower at Oxford University, veterinary transfusion medicine scientifically and technologically lags behind its counterpart in human medicine.76 Information in this chapter is based on veterinary studies whenever possible. When none is available, currently accepted guidelines from human medicine will be applied to the veterinary patient. The purpose of this chapter is to provide the reader with the following:
1. A basic understanding of the theory of blood component therapy
2. Information on the technical aspects of obtaining blood for transfusion
3. Suggestions for the administration and monitoring of transfusions
4. A description of the clinical applications of a veterinary blood substitute
Basics of blood components
Blood is the body’s largest connective tissue. When collected from the donor, it contains all the elements of blood: red blood cells, white blood cells, platelets, coagulation factors, immunoglobulins, and albumin. Whole blood can be transfused into the recipient as it is collected from the donor, but it is neither a specific therapy nor an economical use of blood. The optimal method of preservation of blood for transfusion is to separate whole blood into its component parts. Appropriate use of blood components not only conserves the products but also allows the most specific and safe product to be administered for each patient. When blood components are used instead of whole blood for transfusion, two dogs can benefit from 1 unit of whole blood. A plasma transfusion counteracts the anticoagulant effects of rodenticide intoxication in one dog, and red blood cells from the same donor provides enhanced oxygen-carrying capacity in a second, anemic dog. Component transfusions also have been used in cats, but preparation of components is more difficult because of the small volume of blood collected from donor cats.23,53,68,99
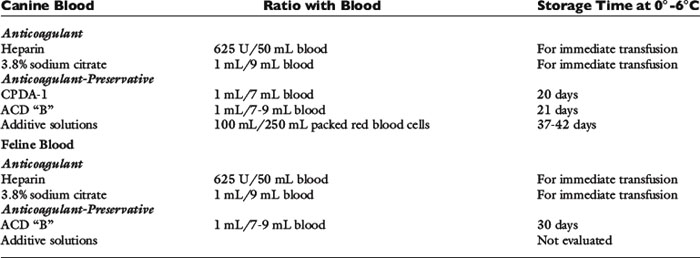
The technical aspects of component production are not included in this chapter but can be found elsewhere.85,99 A brief summary follows. Preparation of blood components from whole blood requires that the blood from the donor be collected into the anticoagulant-containing bag of a multibag plastic blood collection system. The whole blood then is separated into packed red blood cells (PRBCs) and plasma by differential centrifugation in a refrigerated blood bank centrifuge, and the plasma is transferred into one or more of the attached satellite bags via the sterile tubing linking the bags. The bags are separated, and PRBCs are stored in a refrigerator and plasma is stored in a freezer. Blood collected into glass bottles is not amenable to centrifugation and cannot be processed into components. Additionally, storage of canine blood in a glass bottle results in lower levels of 2,3-diphosphoglycerate and adenosine triphosphate (ATP) than blood stored in plastic bags; consequently, plastic bags are the preferred storage container for blood.28
Whole blood
Whole blood is the blood collected from the donor plus the anticoagulant. In veterinary medicine, no standards have been established for the volume of blood that constitutes 1 unit. When a human blood collection system is used for dogs, 450 ± 45 mL of blood is collected and combined with 63 mL of anticoagulant, and often is designated as 1 unit. Whole blood contains red blood cells, clotting factors, proteins, and platelets and is the product most commonly transfused into dogs and cats.56 Once whole blood is refrigerated, the white blood cells and platelets become nonfunctional. As a starting point, the dosage for whole blood is 10 to 22 mL/kg.
Packed red blood cells
PRBCs are the cells and the small amount of plasma and anticoagulant that remains after the plasma is removed from 1 unit of whole blood. If 450 mL of blood are collected, the volume of PRBCs obtained is approximately 200 mL. Because the plasma has been removed, the total volume transfused is less than 1 unit of whole blood but contains the same oxygen-carrying capacity as 450 mL of whole blood. In cats, the increase in packed cell volume (PCV) after transfusion of 1 unit of PRBCs has been shown to be equivalent to the increase after transfusion of 1 unit of whole blood.68 PRBCs are used only to treat clinically symptomatic anemia because they do not contain platelets or clotting factors. Red blood cell transfusions are administered to cats for a variety of reasons. Data on 126 cats administered whole blood or PRBCs indicated 52% were transfused for blood loss anemia, 38% for erythropoietic failure, and 10% for hemolytic anemia.68 Similar reasons for transfusion of cats have been reported elsewhere.22,126 Dogs more commonly are transfused for blood loss anemia (70%) with 14% to 22% being transfused for hemolytic anemia and 8% to 14% for erythropoietic failure.17,64 The initial dosage of PRBCs is 6 to 10 mL/kg, and transfusion is continued until the clinical signs of anemia resolve.
Fresh frozen plasma
Fresh frozen plasma is the plasma obtained from whole blood plus the anticoagulant solution, which is frozen within 8 hours of collection. When whole blood is centrifuged to produce plasma and PRBCs, the anticoagulant segregates with the plasma fraction. Fresh frozen plasma contains all clotting factors, which, if frozen at −30° C in a blood bank freezer, maintains activity for 12 months.121 Fresh frozen plasma maintained in an upright freezer at −20° C maintains clotting factor activity for 6 months. When frozen, the plastic storage bag becomes brittle and if not carefully handled can crack, rendering the plasma unusable. For this reason, plasma is stored in special boxes to protect the plastic bag and must be handled carefully before transfusion. If plasma is thawed, and not transfused, it can be refrozen within 1 hour of thawing without loss of coagulation factor activity.131
Fresh frozen plasma has been used to treat a wide variety of clinical patients. A retrospective analysis of fresh frozen plasma usage in dogs identified multiple indications for administration of fresh frozen plasma, including replacement of coagulation factors, albumin, α2-macroglobulin, and immunoglobulin despite the recommendation that fresh frozen plasma should not be used as a source of albumin, for volume expansion, or nutritional support.75,87 Calculations suggest 45 mL/kg of plasma would need to be given to increase albumin serum concentration by 1 g/dL.120 In cases of coagulation factor deficiencies, plasma should be given to effect (i.e., until active bleeding ceases).70 For the treatment of coagulation disorders, 6 to 10 mL/kg is the recommended starting dosage. Multiple doses may be required to control bleeding because of the short half-life of clotting factors, especially in patients with disseminated intravascular coagulation. Normalization of previously abnormal coagulation tests can be used as a guide for discontinuation of plasma therapy.
Cryoprecipitate
Cryoprecipitate is prepared by thawing fresh frozen plasma at 0° C to 6° C. A white precipitate forms, the liquid plasma is removed after centrifugation, and both aliquots are refrozen. The cryoprecipitate is a concentrated source of von Willebrand’s factor, fibrinogen, and factors XIII and VIII (antihemophilia factor). It is useful in the treatment of deficiencies of these clotting factors and is handled in the same manner as fresh frozen plasma. Two studies have shown cryoprecipitate to be the blood product of choice for the treatment of von Willebrand’s disease because it concentrates the larger, more hemostatically active von Willebrand’s multimers into a smaller volume than fresh frozen plasma.23,108 A preliminary study suggests cryoprecipitate corrects the hypocoagulable state associated with disseminated intravascular coagulation when administered at a dose of 5 to 7 ml/kg.117 Cryoprecipitate is equivalent to fresh frozen plasma for the treatment of hemophilia A. The dose is 1 unit per 10 kg body weight.84
Platelet-rich plasma
Platelet-rich plasma is prepared from fresh whole blood by centrifugation at a slower rate than is used for production of PRBCs and plasma.85 The platelets are suspended in a small amount of plasma to facilitate transfusion. Storage of fresh platelets is impractical outside a blood bank because of their requirement for storage at 20° C to 24° C in special plastic bags and continuous agitation.2 Transfused platelets are rapidly destroyed in human patients with immune-mediated thrombocytopenia, and because immune-mediated thrombocytopenia is a common cause of profound thrombocytopenia in dogs, most cases of thrombocytopenia-mediated hemorrhage may not be amenable to successful platelet transfusion. If a platelet transfusion is given, the dose is the platelets collected from 1 unit of whole blood per 10 kg of body weight.
Cryopreserved canine platelet concentrate
Cryopreserved canine platelets are collected from a single donor via plateletpheresis, and the manufacturer reports one bag contains 1 × 1011 platelets preserved in dimethyl sulfoxide (DMSO).57 The bag also contains a small amount of fresh frozen plasma. Efficacy data on this product have not been published, but the manufacturer recommends this product be used for the treatment of immune-mediated thrombocytopenia. The dose is 1 unit of frozen platelets per 10 kg of body weight. According to the manufacturer, anticipated increase in platelet count is 20,000/ μL 1 to 2 hours posttransfusion. Because the product contains DMSO, it must be infused slowly to prevent bradycardia.
Cryopreserved canine platelet concentrate was compared with fresh platelet rich plasma in the laboratory.48 This study identified decreases in platelet number and function as a result of the freeze-and-thaw process. Platelet number decreased 59% compared with the manufacturer’s reported platelet count and platelets demonstrated multiple features of activation. The impact of cryopreservation on platelet function and number in vitro has not been studied.
Serum
The use of serum has been recommended for the treatment of kittens and puppies with failure of passive transfer. Kittens treated with 5 mL subcutaneously or intraperitoneally three times in 24 hours achieved immunoglobulin G (IgG) concentrations comparable to kittens receiving colostrum.72 Treatment of puppies with 22 mL/kg of serum given orally or subcutaneously at birth did not result in equivalent IgG and IgA concentrations when serum-treated puppies were compared with nursing puppies.90 IgM was higher in the puppies treated with subcutaneously administered serum.
Human albumin
Human albumin is a concentrate of albumin derived from pooled human plasma. Homology between canine and human albumin is approximately 79%, and human albumin is antigenic in dogs.30,80 Previous human albumin transfusion does not appear to be required for production of antibodies in dogs.80
Hypoalbuminemia predicts a negative outcome in several canine diseases; consequently, the ability to correct hypoalbuminemia by albumin transfusion would be a medically desirable intervention.1,20 Because canine albumin was not previously available, human albumin has been used in dogs. Two retrospective studies have evaluated transfusion of human albumin to critically ill dogs.81,115 One associated improved albumin levels and blood pressure with human albumin administration and did not report serious adverse events.81 The second concluded the serious nature of the diseases treated with precluded recognition of complications of the transfusion.115 A recent study performed in normal dogs has identified serious adverse events suggestive of anaphylactic and fatal type III hypersensitivity reactions.25 Transfusion of dogs with human albumin should be undertaken with great caution especially because lyophilized canine albumin is available.58
Intravenous immunoglobulin
Human intravenous immunoglobulin (hIVIG) is a highly purified preparation of immunoglobulin G, obtained from large pools of donated human plasma. The manufacturer provides the product as a lyophilized powder, which is reconstituted before transfusion. Sporadic availability of hIVIG limits its use, as does its high cost. Estimates indicate the cost of the drugs alone may be as high as $3000 to treat a 20 kg dog.129 Reconstituted hIVIG is infused over 6 hours. Most report a single administration of the drug at a dosage of 0.5 to 1.0 g/kg, but in some cases the dose is administered three times on 3 consecutive days.8,9,113,129
Because of its immunomodulatory properties, transfusion of hIVIG has become more common in veterinary patients.94 The two major diseases treated with hIVIG have been immune-mediated hemolytic anemia (IMHA) and immune-mediated thrombocytopenia (ITP), but hIVIG has also been used to treat some immune-mediated dermatologic disorders as well.8,9,113,129 Randomized, controlled prospective studies of glucocorticoids with and without hIVIG for the treatment of IMHA and ITP have been published.8,129 The ITP study demonstrated reduction in platelet recovery time without a concurrent increase in associated charges in the group randomized to receive glucocorticoids and hIVIG. The IMHA study did not show an advantage to treatment with hIVIG and glucocorticoids compared with glucocorticoids alone, but the study was underpowered to distinguish a difference between the two treatment groups. Administration of hIVIG to normal dogs promoted a hypercoagulable state, but in clinically ill dogs causality of thromboembolism is difficult to determine given the complexity of diseases undergoing hIVIG transfusion.100,116
Sources of blood and blood products for transfusion
The most convenient source of blood for a veterinary clinic is a commercial blood bank. Currently, there are only a limited number of commercial veterinary blood banks in the United States, and they cannot adequately supply all the small animal practices in the country with blood (see Box 24-1). Veterinary school blood donor programs may serve as an additional source of blood for the practitioner.56
Because of the limited supply of blood from commercial animal blood banks, small animal practitioners typically borrow a donor from an employee or maintain a blood donor on the premises.56 Borrowing a donor from either an employee or a client is a frequently used, if less convenient, option and is less expensive than maintaining an in-hospital donor. Maintaining a donor on the premises is advantageous because they are readily available for donation and their health status and disease exposure can be controlled, but the expense associated with feeding, housing, and caring for a blood donor is significant.54 Volunteer blood donor programs have replaced many on-site blood donors.14,59 Collecting blood from stray animals is unsafe because infectious disease exposure and health status are unknown.
Blood donor selection
Identification of donor dogs and cats before blood is needed is essential to allow blood type to be determined and the health status of the donor to be assessed before blood collection, thus ensuring the safety of the blood being transfused. The American College of Veterinary Internal Medicine has published recommendations on infectious disease screening for canine and feline blood donors as a consensus statement.124 The recommendations have been incorporated into the following sections.
Dogs
For nearly 60 years, the best blood donor was believed to be a large, quiet dog not requiring anesthesia during blood collection.78 The current recommendation is unchanged. A canine blood donor weighing more than 27 kg can safely donate 450 mL of blood in one donation, allowing collection of blood into commercially manufactured blood collection bags designed to facilitate sterile processing of components. Dogs weighing 27 kg or more have been shown to consistently donate 1 unit of blood for 2 years at 3-week intervals.92 Dogs selected as donors also should have an easily accessible jugular vein to facilitate venipuncture. Prior pregnancy does not exclude female dogs from donation because pregnancy does not induce alloantibodies.12
Greyhounds have been promoted as ideal blood donors because of their gentle disposition, high hematocrits, and lean body type, which simplifies blood collection.45 Many greyhounds are euthanized because of poor racing performance, and these dogs are available from racetracks, breeders, and rescue organizations.36
Blood banks choosing greyhounds as blood donors should be aware of certain breed idiosyncrasies that will impact on the management of a greyhound donor. The greyhound idiosyncrasy most important in transfusion medicine is the high red blood cell count, PCV and hemoglobin concentration, and low white blood cell counts and platelet count compared with mixed breed dogs.91,109 Greyhounds in Florida have a 46% seroprevalence of babesiosis.111 Because the geographic origin of greyhounds serving as blood donors cannot always be determined, all greyhounds being screened as donors should have serologic testing for Babesia canis performed, and dogs with positive titers should be excluded as donors. Greyhounds with negative titers against B. canis should have B. canis polymerase chain reaction (PCR) performed, and if the test is positive, the dog should be excluded as a donor.
In addition to the tendency of greyhounds to be asymptomatic carriers of B. canis, some other breeds of dogs should be used cautiously as blood donors because they are known to be asymptomatic carriers of infectious organisms transmitted by transfusion. American pit bull terriers and Staffordshire bull terriers recently have been recognized as carriers of Babesia gibsoni.10,77 Use of these dogs as blood donors should be restricted to those dogs that are seronegative and PCR-negative for B. gibsoni. Leishmaniasis has been identified in American foxhounds and transfusion of Leishmania infantum-infected blood from American foxhounds resulted in clinical leishmaniasis in transfusion recipients.47,89 All potential foxhound donors should be screened for Leishmania sp.
Determination of blood type is critical to the selection of a blood donor dog. Although seven canine blood groups or blood type systems have received international standardization, typing sera are available for only five types (Box 24-2). Red blood cells can be negative or positive for a given blood type, except for the dog erythrocyte antigen (DEA) 1 system, which has three subtypes: DEA 1.1, 1.2, and 1.3. Canine red blood cells can be negative for all three subtypes (a DEA 1-negative blood type) or positive for any one of the three subtypes. Naturally occurring alloantibodies occur infrequently and without previous sensitization from transfusion do not appear to cause transfusion incompatibility in the dog51 (see Box 24-2). A new canine red blood cell antigen, Dal, has recently been described.11 This antigen appears to be common in the general canine population and lacking in Dalmatians. Transfusion with Dal-positive blood, induced an anti-Dal antibody producing multiple incompatible crossmatch tests. Dogs producing anti-Dal antibodies are at risk for hemolytic transfusion reactions.
The blood type of the ideal canine blood donor is not uniformly agreed on among transfusion experts. Of the five blood groups for which typing sera are available, a transfusion reaction has been attributed to antibodies against DEA 1.1 induced by a DEA 1.1-positive transfusion in a DEA 1.1-negative recipient and to an antibody induced by a DEA 4-positive transfusion in a DEA 4-negative dog.40,83 In theory, red blood cells expressing DEA 1.2 can sensitize a DEA 1.2-negative transfusion recipient, resulting in an acute hemolytic transfusion reaction if a second transfusion of DEA 1.2-positive blood is given. In a laboratory setting, antibodies against DEA 1.2 have been reported to cause transfusion reactions, but clinical reports of hemolytic transfusion reactions mediated by anti-DEA 1.2 antibodies are lacking. DEA 7 is believed to be structurally related to an antigen found in common bacteria. A naturally occurring antibody against DEA 7 has previously been described in 20% to 50% of DEA 7-negative dogs, but recently revised down to 9.8% of dogs.51 This antibody may result in accelerated removal of DEA 7-positive cells from a DEA-negative donor with anti-DEA 7 antibodies.51,102 Based on this information, the recommendation has been made to select donors that are negative for DEA 1.1, 1.2, and 7. Others suggest the donor dog should also have red blood cells positive for DEA 4 to be designated as a universal donor.50 The recent description of a transfusion reaction attributed to antibodies against DEA 4 in a dog with DEA 4-negative red blood cells calls into question this recommendation.119 Ninety-eight percent of dogs are DEA 4-positive, making it easy to find donors of this blood type. The importance of DEA 3 and 5 and Dal in blood donor selection remains to be determined.
Cats
It is essential to determine the blood type of potential donors. One feline blood group system has been identified with three blood types: A, B, and AB (see Box 24-2) and recently a new common red blood cell antigen, Mik has been identified.6,128 Unlike dogs, cats have naturally occurring alloantibodies. Type A cats have naturally occurring alloantibodies against type B cells and type B cats against type A cells.38 Cats of blood type B have strong hemagglutinating antibodies of the IgM type against type A cells, and cats of blood type A have weak hemolysin and hemagglutinating antibodies of the IgM and IgG type against type B cells. The clinical significance of these alloantibodies is threefold in transfusion medicine. First and most importantly, a cat may have a transfusion reaction without sensitization from a previous transfusion; second, type A kittens born to a type B queen are at risk for neonatal isoerythrolysis21; and third, the antibodies are useful in determining the blood type of a cat. Mik appears to be a common red blood cell antigen. Only a few cats lacking Mik have been identified and they all produce anti-Mik alloantibodies.128
Donors of both type A and type B blood must be available because there is no universal donor in cats. Incompatible transfusions result in shortened red blood cell survival and potentially death in the transfusion recipient; therefore the serologic compatibility between recipient and donor must be determined before every transfusion in cats.38 Donors of type A blood are easy to find because more than 99% of the domestic cats in the United States are type A.42 The prevalence of domestic cats with type B blood varies geographically. In the United States, the western states have the highest percentage of type B cats, 4% to 6%.42 Australia has the highest reported percentage of type B cats in their domestic cat population, 73%.6 In Europe, the frequency of blood type B in domestic cats varies from 0% in Finland, 14.9% in France, and 24.6% in Turkey.3,41 Some purebred cats have a higher frequency of type B in their population.39 The British shorthair, Devon rex and Turkish van have been reported to have the highest proportion of type B individuals, approximately 50% to 60%.4 The Siamese, Oriental shorthair, Burmese, Tonkinese, American shorthair, and Norwegian forest cat breeds have not been reported to have any members with type B blood. Blood type AB is extremely rare, occurring in 0.14% of cats in the United States and Canada.46 Fortunately, a type AB donor is not required to successfully transfuse a type AB cat. Blood from a type A cat is adequate.
Blood donor screening for infectious disease
Screening blood donors for infectious diseases transmitted by blood transfusion is an integral step in maintaining a safe blood supply. Infectious disease screening of canine and feline blood donors varies within the different geographic regions of the United States and with the breed of the blood donor. An American College of Veterinary Internal Medicine consensus statement, developed by a committee consisting of members of the Infectious Disease Study Group and the Association of Veterinary Hematology and Transfusion Medicine should serve as the guideline for donor screening.124
Organisms infectious to dogs and known to be transmitted by blood transfusion include B. canis, B. gibsoni, Haemobartonella canis, and Leishmania sp.31,71,89,105 All canine blood donors should be screened for Ehrlichia canis and Brucella canis, and if they test positive, they should be eliminated from the donor pool. Titers against E. canis less than 1:80 may be false positives and should be repeated in 2 to 3 weeks. Dogs with initially negative titers to E. canis can receive additional screening with a PCR test. Splenectomy of donor dogs to facilitate identification of B. canis and H. canis carriers is not recommended. In neutered dogs, a single negative test for Brucella canis is adequate. Based on travel history and breed, additional screening for Trypanosoma cruzi, Bartonella vinsonii, B. canis, B. gibsoni, L. donovani, and organisms previously classified as Ehrlichia spp. (Anaplasma phagocytophilum and Anaplasma platys) may be indicated.124 Dogs should not donate if they are ill or have fever, vomiting, or diarrhea; using donors with these clinical signs has resulted in Yersinia enterocolitica contamination of human units of blood.32
Organisms infectious to cats and known to be transmitted by blood transfusion include: feline leukemia virus (FeLV), feline immunodeficiency virus (FIV) Bartonella henselae, Anaplasma phagocytophilum, Ehrlichia spp. and Neorickettsia spp., and the organisms formerly classified as Haemobartonella sp. (Mycoplasma haemofelis and Candidatus Mycoplasma haemominutum).33,49 Potential donor cats should be screened for FeLV and FIV. Because the prepatent period for FeLV infection can be 3 months, cats being considered as donors should be screened monthly for FeLV for 3 consecutive months. Testing for FIV antibodies can be performed simultaneously. Bartonella henselae is an emerging feline infectious disease and has been transmitted to cats by infected blood.69 The use of cats with positive serology or cultures for B. henselae as blood donors is controversial and eliminating these cats from the donor pool is the safest approach. Testing for hemoplasma should include both light microscopy and PCR, and infected cats should be eliminated from the donor pool.33 Screening of donor cats for feline infectious peritonitis (FIP) is problematic because there is no reliable test to identify the FIP-causing coronavirus. Feline blood donors should be screened for infection with Cytauxzoon felis and the agents causing feline ehrlichiosis if they reside in or are known to have traveled to endemic locations.
Blood donor health maintenance
A safe blood supply begins with healthy blood donors. All blood donors should undergo a complete physical examination each time they donate blood. Complete and differential blood counts, biochemical profile, and fecal examination should be performed annually. Donor cats and dogs with exposure to the outdoors or to ectoparasites should be routinely screened for infectious disease. Blood donors should be tested for heartworms, treated for ectoparasites, and vaccinated for the diseases on the schedule recommended for pets residing in the geographic region of the blood bank. Because the ideal feline blood donor lives in an indoor environment and is not exposed to other cats, the author believes vaccinations against FeLV, FIV, and FIP are unnecessary in donor cats. Exposure to the outdoors or to fleas approximately doubles the prevalence of hemoplasma infections in donor cats and restricting access to the outdoors, fleas, and other cats can prevent most infectious diseases in feline blood donors.49
Equipment and supplies for collection of blood
Skin preparation
Strict aseptic technique must be used during the blood collection process to prevent contamination of blood with microorganisms. Whenever possible, solutions and equipment used for the collection process should be single-use products to prevent inadvertent contamination of blood.55 After clipping the hair over the venipuncture site, the skin is surgically scrubbed. The ideal skin preparation regimen is yet to be determined in animals; however, in human blood donors, a 30-second, 70% isopropyl scrub followed by a 2% iodine tincture resulted in better skin surface disinfection than alcohol followed by chlorhexidine or green soap.44 The phlebotomist wears sterile surgical gloves and performs venipuncture without touching the scrubbed area.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree