Fig. 22.1
Representative light micrograph (a) and confocal laser scanning micrographs (b–d) showing periodic acid-Schiff staining ( PAS ; a), its fluorescence emission ( PAS fluorescence emission; b), and immunofluorescence staining for zonula occludens-1 (ZO-1; c) on the same section prepared by “in vivo cryotechnique ” under the normotensive condition. The layer at glomerular basement membrane positive for PAS fluorescence emission mostly surrounds the glomerular capillary loop s and closely localizes near the foot process layers visualized by the ZO-1 immunostaining (d, arrows and arrowheads). Gl glomeruli, PT proximal tubule s , asterisks blood capillaries . Scale bars 10 μm
22.3 Alternation of Serum Protein Distribution in Glomeruli Under Various Hemodynamic Conditions
As PAS fluorescence emission has been well identified to represent GBM , the distribution of serum protein s in living mouse glomeruli could be estimated by comparing the immunolocalization of serum proteins and GBM using PAS fluorescence emission combined with IVCT. Serial sections of kidneys under different hemodynamic condition s were prepared with IVCT and immunostained to detect the distribution of albumin , immunoglobulin G heavy and light chains (IgG (H+L)), as well as the kappa light chain and IgG1 heavy chain s (IgG1) (Fig. 22.2).
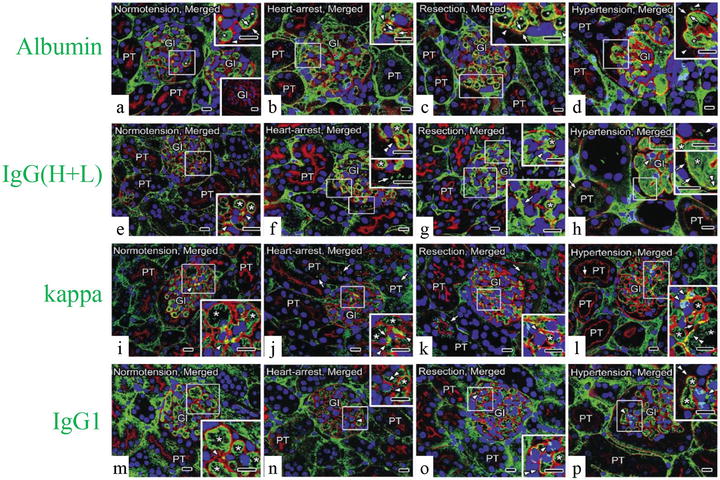

Fig. 22.2
Representative confocal laser scanning micrographs showing the alternation of serum protein (green color) distribution in living mouse kidney under various hemodynamic condition s using PAS fluorescence emission (red color). Nuclei are labeled with TO-PRO-3 (blue color). Under the normotensive condition (normotension), the immunolocalization of albumin (a) is primarily restricted within glomerular capillary loop s (GCL; arrowhead) and slightly overlapped with the PAS fluorescence emission at glomerular basement membrane ( GBM ; arrows); immunolocalizations of immunoglobulin G high and light chains (IgG (H+L), e), the kappa light chain (kappa, i), and IgG1 heavy chain (IgG1, m) are detected within GCL and at some mesangial areas (MA; arrowheads). Under the heart-arrest condition (heart arrest) and in the kidney tissues quickly frozen after tissue resection (resection), the GCL are collapsed, and the leaked albumin is additionally immunolocalized in Bowman’s space (b and c, arrowheads); immunoreaction products of IgG (H+L) are also localized in Bowman’s space (f and g, arrows); kappa are clearly localized in Bowman’s space and also proximal convoluted tubule s (j and k, arrows); IgG1 are localized at MA (n and o, arrowheads), but neither in Bowman’s space nor in proximal convoluted tubules. Under the acute hypertensive condition (hypertension), albumin is more clearly immunolocalized in Bowman’s space (d, arrowheads); IgG (H+L) immunostaining is clearly detected in Bowman’s space and also in proximal convoluted tubules (h, arrows); kappa is immunolocalized in Bowman’s space (l, right upper inset, arrows), the MA (arrowhead), and the apical cytoplasm of some proximal tubule s (arrows); IgG1 is detected exclusively within the GCL and at some MA (p, arrowheads), but not in Bowman’s space. In addition, under the heart-arrest or acute hypertensive conditions and in the kidney tissues quickly frozen after tissue resection, the glomerular areas, where the immunoreactivity of albumin (b–d), IgG (H+L) (f–h), kappa (j–l), and PAS overlapped each other (arrows), respectively, appear to become wider than those under the normotensive condition (a, e, i, arrows), whereas the overlapping of IgG1 and PAS appears to be unchanged (m–p, double arrowheads). Gl glomeruli, PT proximal tubules, asterisks blood capillaries . Scale bars 10 μm
Under normal hemodynamic condition , both serum albumin and IgG were almost kept within GCL (Fig. 22.2a, e), and their immunoreactivity was more widely overlapped with the PAS fluorescence emission under the acute hypertensive condition (Fig. 22.2d, h). Although the main filtration barrier was reported to be GBM itself by electron microscopy [5, 21], other reports also demonstrated that slit diaphragms of foot process es formed the ultimate filtration barrier for macromolecular permeability [22, 23]. Considering these previous reports, our findings suggest that more serum protein s reached the outer layer of GBM under the acute hypertensive condition and the slit diaphragms would play a significant role especially in such pathological or physiological states. The translocation of serum proteins may be due to the acutely increased pressures in the GCL, and if the blood pressures were temporarily so high, lots of serum proteins would leak out into Bowman’s space.
Under abnormal conditions, the leakage of albumin (Fig. 22.2b–d) and kappa light chain (Fig. 22.2j–l), but not IgG1 (Fig. 22.2n–p), was clearly detected in Bowman’s space. Serum proteins with low molecular weights, such as albumin and kappa light chains, could easily translocate to the urinary space and reabsorbed in the convoluted proximal tubule s [24–27]. On the other hand, larger or cationic molecules, such as IgGs, were trapped in the GBM and hardly leaked out into Bowman’s spaces under some physiological or pathological conditions, such as membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis [28–30]. The differences of protein immunolocalization are undoubtedly due to the size and charge selectivity of the GBM [31].
In the present experiment, the overlapping of PAS fluorescence emission with the IgG (H+L) immunoreactivity close to Bowman’s space appeared to be prominent at some parts on one side of the GCL under the acute hypertensive condition (Fig. 22.2h). As such heterogeneity of leaking areas was also observed using extrinsic tracers [21], the leakage degree of serum protein s would exhibit their heterogeneous immunolocalizations not only among glomeruli but also among GCL under the acute hypertensive condition.
The leakage of both albumin and IgG (H+L) through GBM into Bowman’s spaces was detected under the heart-arrest condition (Fig. 22.2b, f) or with the quick-freezing of the resected kidney tissues (Fig. 22.2c, g), which might be caused by low blood pressures and ischemia , resulting in serious alteration of GCL structures and molecular organization [5, 32]. Moreover, the ischemia or hypoxia , including the hypotensive condition, was reported to induce changes of the glomerular structures and also damages of intercellular junctions in renal vasculatures, resulting in the increased vascular permeability and local interstitial edema of kidneys [7, 33–35].
22.4 Concluding Remarks
In conclusion, the fluorescence emission of PAS staining allowed us to examine the precise immunolocalization of serum protein s at the GBM under various hemodynamic condition s of living mouse kidney s in combination with the IVCT followed by freeze substitution. In addition, the IVCT would be a reliable tool to observe soluble serum protein s in situ and capture transient images of functioning glomeruli in the living mice. Further detailed analyses, e.g., by using immunoelectron microscopy in conjunction with the “in vivo cryotechnique ,” would enable us to characterize the changes of soluble serum proteins around GBM, which may be new findings of clinical importance. The present figures were already published in our paper, Arch Histol Cytol (2006) 69:147–161, and cited with their permissions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


