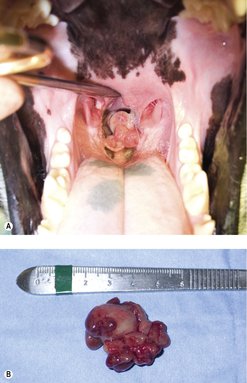
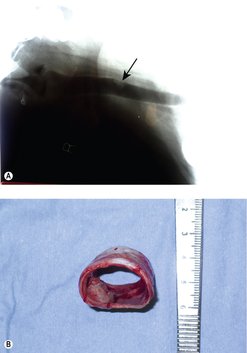
Laryngeal tumours
Cancers of the laryngeal region are rare in both dogs and cats. No breed predilection has been seen in either species. Benign laryngeal oncocytomas (rhabdomyomas) occur infrequently in young dogs (Meuten et al 1985, Pass et al 1980). Other types of benign laryngeal tumours include lipomas, leiomyomas, chondromas and osteochondromas.
Malignant tumours of the canine larynx include squamous cell carcinoma (SCC), adenocarcinoma, chondrosarcoma (CSA), osteosarcoma (OSA), rhabdomyosarcoma, mast cell tumours (MCT), plasma cell tumours and lymphoma (LSA) (Brodey et al 1969, Carlisle et al 1991, Crowe et al 1986, Flanders et al 1987, Hayes et al 2007, Saik et al 1986, Wheeldon et al 1982).
In cats, LSA and carcinomas are the most common tumours of the larynx (Jakubiak et al 2005, Vasseur 1981, Wheeldon & Amis 1985).
Tracheal tumours
Cancers of the tracheal region are also rare in both dogs and cats. In the former, the most common types are chondromas, osteomas, osteochondromas or carcinomas; young dogs can develop osteochondromas at less than 1 year of age that may be surgically excised with a good prognosis (Carb & Halliwell 1981, Dubielzig & Dickey 1978, Hough et al 1977).
Other benign tumours seen include leiomyomas and polyps (Black et al 1981, Bryan et al 1981). Uncommon canine tracheal malignancies include plasmacytomas, OSA, CSA, MCT, LSA, SCC and rhabdomyosarcoma (Chaffin et al 1998, Henderson et al 1991). In the cat the most commonly reported tracheal tumour is LSA; malignant epithelial and mesenchymal tumours have also been reported (Bell et al 2006, Evers et al 1994,Kim et al 1996, Lobetti & Williams 1992, Rossi et al 2007, Schneider et al 1979).
Neoplasia of thyroid gland, oesophagus, lung or aortic chemoreceptor may also invade the trachea.
Clinical signs
Dyspnoea, wheezing, coughing and stridor (may be inspiratory and expiratory) are the usual presenting signs (usually progressive). Occasionally, dysphagia and weight loss will be the major clinical signs. With laryngeal tumours changes in phonation may be noticed, and a mass may be palpable in the pharyngeal area. Clinical signs would be expected to worsen with excitement, stress, hot weather or exercise. Cats may cope better and for longer than dogs, as they are generally less active and excitable.
Diagnostic work-up
Radiographs will usually reveal a distinct mass or a narrowing of the lumen of the trachea around the laryngeal region. Laryngoscopy or tracheoscopy allows good visualization and sufficient access for biopsy in most cases. The anaesthetist should be prepared for an emergency temporary tracheostomy if normal laryngeal intubation is not possible. Biopsies should be taken (incisional) and impression smears made for cytology as well as tissue sent for histopathology. A single incisional biopsy was diagnostic in 22 of 27 cats (Jakubiak et al 2005).
Thoracic radiographs may be indicated for staging of disease, and may show signs secondary to upper respiratory tract obstruction (e.g. pulmonary oedema, pleural effusion, pectus excavatum). Regional lymph nodes (RLN) should be palpated for enlargement. Displacement of the local structures of the pharyngeal, neck or intrathoracic regions may be appreciated with further imaging, e.g. CT/MRI or radiographs.
Treatment options
Laryngeal tumours
Surgery
Benign laryngeal tumours (e.g. rhabdomyomas or oncocytomas) may be resected with preservation of normal laryngeal function, and have a good prognosis. In most malignant laryngeal cancers (Figure 14.1), total laryngectomy and permanent tracheostomy are required in the attempt to remove all local disease. One dog undergoing total laryngectomy and permanent tracheostomy for a laryngeal rhabdomyosarcoma had no evidence of disease 18 months after surgery (Block et al 1995). Pharyngeal dysphagia, local/distant tumour recurrence, care of a permanent tracheostomy wound, short- and long-term morbidity to the animal are factors for the client and clinician to consider carefully.
Radiation therapy and chemotherapy
If the tumour has a known responsiveness to chemotherapy or radiation therapy (e.g. if the tumour is of lymphoid origin), then this may be the best treatment option. However, it is important to obtain a definitive histopathological diagnosis prior to treatment. An incisional biopsy may also be therapeutic if it assists in the relief of upper respiratory tract obstruction. However, in cases of severe obstruction, an emergency debulking surgery may be required. This may allow initial palliation of clinical signs and provides tissue for diagnosis. It may be followed with adjuvant chemotherapy or radiotherapy, if considered beneficial.
For tumours not amenable to surgical excision without unacceptable morbidity as judged by the clinician and/or the client, surgical debulking and/or medical management may be indicated. Malignant laryngeal tumours generally have a poor prognosis, regardless of treatment.
Tracheal tumours
Surgery
Many tracheal tumours are benign and have a good prognosis with complete excision (Figure 14.2).
Surgery generally involves the resection of affected trachea and end-to-end anastomosis of tracheal rings. Tension is more of a problem in puppies than in adult dogs. Tension-free anastomosis is achievable if no more than 25–50% of the tracheal length (five or six tracheal rings) is removed in adult dogs and 20–25% in puppies (Nelson 2003). A simple continuous suture pattern is recommended (Demetriou et al 2006).
Tracheal wall replacement with latissimus dorsi (deficit <30% of ventral tracheal wall, <50% of dorsal tracheal wall and limited to five tracheal rings or less) has been successful (Fujita et al 1987). If fewer than five rings or >50% of the trachea is removed, reconstruction becomes more problematic. Rigid structures such as pedicled costal cartilage and rib, free cartilage, polypropylene mesh, collagen grafted mesh, mucous membrane grafted mesh, silicone rubber or non-porous silastic tubing can be used (Eckersberger et al 1987, Goldstein et al 1987, Kim et al 2004, Klopper 1969, Okumura et al 1994, Suh et al 2001, Yamato 1992).
Polytetrafluoroethylene grafts did not work in one study (Cull et al 1990) and jejunal autografts, with or without metal stenting, are sometimes successful but mostly unrewarding (Letang et al 1990, Ma et al 1990, Nakayama 1990, Szántó et al 2001).
Other treatment options
These include radiation, chemotherapy, endoscopic removal/debulking and photodynamic therapy. Chemotherapy is beneficial for tracheal LSA.
TUMOURS OF THE MEDIASTINUM
However, a number of other tumours and structures can also be seen, e.g.
• thymic carcinoma
• branchial cysts
• ectopic thyroid tissue
• chemodectoma
• metastatic disease
• other rare tumours, e.g. HSA, mesotheliomas
• local extension of rib or sternal tumours into the mediastinum.
Clinical signs
Clinical signs are usually respiratory in nature due to the space-occupying effect of a mass that can be of considerable size (Figure 14.3A). A pleural effusion may or may not be present. The most common presenting clinical signs are exercise intolerance or acute respiratory distress, and in some cases the patient can present with regurgitation due to compression of the oesophagus.
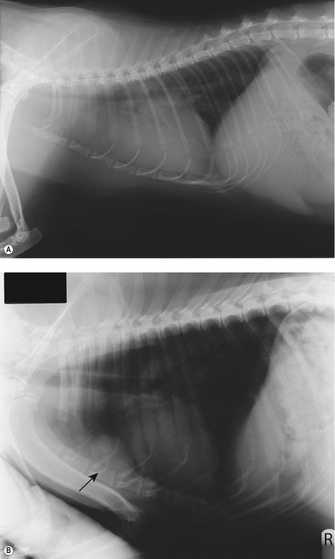 |
| Figure 14.3 |
Precaval syndrome can be associated with mediastinal disease due to the obstruction of venous or lymphatic drainage from the head, neck and forelimbs. Paraneoplastic syndromes (PNS) are common with tumours in this region, especially LSA (e.g. hypercalcaemia) and thymomas (regurgitation) and often it is the paraneoplastic syndromes that alert the client to the fact there is a problem (Figure 14.3B).
Diagnostic work-up
For patients presenting with respiratory problems, precaval syndrome or regurgitation, thoracic radiographs are indicated (good quality right and left lateral and dorsoventral views). A cranial mediastinal mass may be accompanied by dorsal elevation of the trachea and oesophagus, caudal displacement of cardiac silhouette (depends on size) ± aspiration pneumonia, pulmonary metastasis (unusual) and pleural effusion (if invasive).
Patients presenting with other clinical signs may start off with a different work-up, e.g. for polyuria/polydipsia (PU/PD) routine blood work and urinalysis would be indicated, but any patient with documented hypercalcaemia requires thoracic radiographs. Haematology and biochemistry profiles are required in all patients with mediastinal masses as part of the diagnostic evaluation.
Obtaining a definitive diagnosis after confirmation of a mediastinal mass on radiographs is important because the definitive therapy for the major two differentials is completely different. Surgery is the treatment for thymoma, whereas chemotherapy is the major treatment for LSA.
How can we distinguish thymoma from lymphoma?
Signalment?
• Cats: Usually older cats have thymoma; younger cats have thymic lymphoma.
• Dogs: Thymoma is typically seen in older dogs; younger dogs are more likely to have lymphoma.
Clinical signs?
These can be similar for both tumours; however, PU/PD is more common with LSA, and regurgitation secondary to megaoesophagus is seen more frequently with thymomas.
Radiographic appearance?
As a mass in the cranial mediastinum, thymoma and lymphoma can be indistinguishable. The presence of other enlarged lymph nodes or abnormalities with other organ systems may indicate the origin of the mediastinal mass.
Presence of PNS?
Fifty per cent of dogs with mediastinal LSA are hypercalcaemic, whereas hypercalcaemia is less likely with thymoma. However, ultimately a tissue sample is required as paraneoplastic syndromes are not exclusive to one tumour type.
Additional diagnostic tests
Thoracic ultrasound
Ultrasound assists not only in delineating the size and echogenicity of a mass, but will also determine whether there is one or more masses in the mediastinum, i.e. the presence of metastatic disease within the mediastinal lymph nodes. It also facilitates diagnosis as fine needle aspirates (FNA) or tru-cut biopsies under ultrasound guidance can often be carried out.
Ultrasound can have difficulty in determining the full extent of a tumour regarding its invasiveness. Thymomas are often of mixed echogenicity and cavitated/cystic (compared to more homogeneous thymic LSA). Because they are cystic, thymomas may need a wedge biopsy for diagnosis.
CT/MRI scan
A CT or MRI scan can be very useful in determining the full extent and invasive nature of a tumour and assist the surgeon in presurgical planning. Wherever possible, a presurgical diagnosis is desired and this is usually attempted either by cytology or tru-cut biopsy.
Other diagnostic tests: thymomas
These include anti-acetylcholine receptor (ACh receptor) antibody titres (serology), Tensilon test to monitor for resolution of myasthenia gravis and immunohistochemistry (cytokeratin).
Thymoma
Thymomas are rare in both the dog and cat, with a median age of 9 years in the dog and 10 years in the cat. No breed or sex predilection has been noted in either species. Thymomas are usually slow-growing tumours and can therefore reach a considerable size before clinical signs are apparent.
Thymomas in dogs are located within the cranial mediastinum. They originate from the thymic epithelium and it is the epithelial component that is neoplastic. They are variably infiltrated with mature lymphocytes and variable numbers of mast cells may also be present. A number of histological types are recognized: lymphocyte-rich, differentiated epithelial and clear cell (Atwater et al 1994). Cytological confirmation of a thymoma rests on the presence of mature lymphocytes, mast cells and epithelial cells (Atwater et al 1994).
In many cases only lymphocytes are seen, making it difficult in some instances to rule out LSA. For this reason, cytology results are often not definitive and should be interpreted with caution. Results of cytology after treatment with corticosteroids or chemotherapy can be even more misleading. A definitive diagnosis (with histopathology via needle core or wedge biopsy if necessary) should be sought prior to initiating any therapy. The definition of benign versus malignant resides primarily in resectability rather than histological features (Withrow 2007). Metastasis was reported in 3 of 14 cats (to RLN and lung) (Patnaik et al 2003).
Treatment of thymomas
• Surgery
• Radiation
• Chemotherapy
Surgery
Surgery is the treatment of choice for non-invasive thymomas, and can be curative (Figure 14.4).
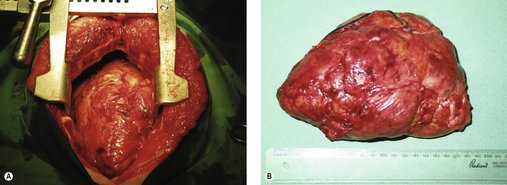 |
| Figure 14.4 (Courtesy R Straw.) |
For invasive thymomas, venous grafts can be performed for invasion of the cranial vena cava. For non-invasive thymomas, careful sharp/blunt dissection, with care of nerves and vessels, often allows removal. Both require thoracotomy via median sternotomy.
Resectability cannot be predicted or assessed until surgery. Size is not an indicator of resectability, and non-invasive thymomas can reach a very large size. Invasive thymomas will be adherent to surrounding tissues, including major vessels, nerves, trachea and oesophagus. Debulking an invasive tumour may alleviate symptoms and enhance the effectiveness of adjuvant treatments.
Radiation
There are few documented cases of radiation treatments in patients with incompletely resected thymomas. Thymomas are moderately radiation sensitive and with appropriate planning there is a role for radiation. Palliative radiotherapy would be an option in patients that were seen as poor surgical candidates due to other medical problems or PNS. For patients with large tumours, presurgical radiotherapy will reduce the size of the tumour very quickly, thus reducing respiratory distress associated with a large-space occupying lesion in the mediastinum. Typically, a single fraction of 800 cGy is sufficient and surgery can then be scheduled within 5–7 days of treatment. Radiotherapy is applicable for those tumours deemed unresectable by surgery.
Adjuvant radiation (combined with incomplete resection) has an increased survival time compared with incomplete resection alone. Complications include pneumonitis and pericarditis.
Chemotherapy
Although rarely considered in veterinary medicine, chemotherapy may be beneficial in patients that are not good surgical candidates and where radiation is not available.
Prednisolone may provide symptomatic relief by reducing the percentage of small lymphocytes in lymphocyte-rich thymomas. In humans, combination protocols utilizing doxorubicin/cisplatin/cyclophosphamide have been used. A definitive diagnosis of thymoma versus LSA versus other cancer should be sought before starting chemotherapy.
PNS and thymomas
A number of PNS have been reported in veterinary patients with thymomas. Myasthenia gravis has been reported in up to 40% of dogs (Atwater et al 1994) and is reported in cats (Gores et al 1994). The typical presentation is of muscle weakness and/or megaoesophagus, and 20–40% of patients have concurrent non-thymic neoplasms, autoimmune diseases, anaemia or polymyositis (Aronsohn et al 1984, Carpenter & Holzworth 1982).
If myasthenia gravis is present it requires treatment, because even if the tumour is treated successfully the symptoms may not resolve. Treatment comprises immunosuppression with prednisolone and the use of anticholinesterase drugs. Symptomatic treatment for megaoesophagus includes antacids ± antibiotics for aspiration pneumonia etc. Other syndromes usually resolve with adequate treatment of the thymoma.
Prognosis
Cats have an excellent prognosis when treated with surgery or radiation therapy. The median survival time with surgery is 21 months, and with radiation therapy is 24 months (Kaser-Hotz et al 2001, Smith et al 2001).
For dogs, the prognosis is excellent for non-invasive thymomas without megaoesophagus. When treated with surgery, these dogs have an 83% 1-year survival and often die of unrelated disease. The prognosis is guarded for invasive thymomas or if megaoesophagus is present (median survival time (MST) 4 days; with myasthenia gravis 67% died within 1 week) (Atwater et al 1994).
Dogs have a higher proportion of invasive thymomas compared to cats, which reflects on the overall improved survival of cats treated with surgery (MST approaching 2 years) (Gores et al 1994). If the tumour is not resectable, then prognosis is poor but survival times for these patients would undoubtedly be improved if they were considered for other treatment options, particularly palliative radiation. The MST for dogs treated with radiation therapy was 248 days (Smith et al 2001).
A recent study (Zitz et al 2008) reported even longer survival times in both cats and dogs treated with surgery alone (MST for cats 1825 days and for dogs 790 days).
Lymphoma of the mediastinum
This was the most common thymic tumour in one study in both cats and dogs (Day 1997). It is less circumscribed than thymoma, with local extension through the thoracic inlet around the heart base or into lung, pericardium, intercostal or cervical muscles.
Feline lymphoma
The classic feline patient with mediastinal lymphoma is a young cat (<2 years of age) that is feline leukaemia virus (FeLV) positive and has a poor prognosis even with doxorubicin-based chemotherapy (Peaston & Maddison 1999). The second peak is seen in older cats that are FeLV-negative and as an isolated form with a good prognosis (Teske et al 2002).
With the control of FeLV due to vaccination we now see cats of all ages with mediastinal lymphoma that are FeLV-negative. FeLV-negative cats younger than 4 years may have a particularly favourable prognosis when treated with multi-agent chemotherapy (Malik et al 2001). Young Siamese cats seem to be over-represented (Court et al 1997, Day 1997, Gabor et al 1998, Louwerens et al 2005, Peaston & Maddison 1999, Teske et al 2002).
Diagnosis is via clinical examination (non-compressible cranial thorax), radiographs (cranial mediastinal mass), cytology (thoracentesis or FNA of mass shows blasts rather than small mature lymphocytes), and biopsy if cytology is not definitive. Thoracic ultrasound often shows a homogeneous and hyperechoic mass compared to mixed echogenicity/cystic thymoma (Konde & Spaulding 1991).
Treatment
Chemotherapy
The majority of patients with mediastinal lymphoma are treated with combination chemotherapy (see Chapter 22 for protocols) and the overall prognosis depends on signalment, FeLV status and protocol.
Radiotherapy
Radiotherapy has a number of indications in the management of feline LSA. Due to the exquisite sensitivity of lymphoid tissue, radiotherapy can be used in the emergency situation when a patient is in significant respiratory distress. Relief within a few hours is possible. Radiotherapy can also be used for patients that are poorly responsive to chemotherapy, especially when they have undergone a relapse. Resistance to chemotherapy does not mean that there will be resistance to radiotherapy. Typical protocols include 5–8 Gy for up to three to four treatments (see Chapter 22).
Canine lymphoma
This is seen more commonly, although not exclusively in young dogs. No breed predisposition has been noted. The most common clinical presentations are respiratory signs, exercise intolerance, PU/PD or vomiting.
Mediastinal lymphoma accounts for ~5% of canine lymphoma (Theilen & Madewell 1987) and 20% of dogs with multicentric lymphoma have cranial mediastinal involvement (Starrak et al 1997). T-cell phenotype is more frequent; when seen in conjunction with hypercalcaemia the overall prognosis is guarded, with MST in the range of 6 months with combination chemotherapy (see Chapter 22).
Radiotherapy in canine mediastinal lymphoma has the same applications as in the feline counterpart and is used only in the emergency situation or when chemotherapy has failed as the first-line approach.
CHEST WALL TUMOURS
Malignant tumours of the chest wall are generally sarcomas. They usually arise from the ribs but can be sternal in origin. Benign tumours (osteomas and chondromas) and infection of bone or soft tissues are differential diagnoses for malignant tumours; however, the clinician should bear in mind that neoplasms can become necrotic or secondarily infected. Most commonly a mass on the chest wall is seen or palpated, and confirmed by radiographs. CT is useful to determine extent of local disease and resectability, and for the presence of pulmonary or lymph node metastasis.
A well-placed and well-executed incisional biopsy (see Chapter 5) is imperative prior to any attempt at surgical resection of a chest wall mass, for two reasons.
1. The prognosis depends greatly on the histological diagnosis, i.e. the client should know their pet’s prognosis prior to embarking on a ‘big’ surgery (although en bloc resection of chest wall tumours involves minimal patient morbidity when performed by an experienced surgeon) (Baines et al 2002).
2. Smaller/debulking type surgeries only worsen the outcome and prognosis by compromising a potential curative resection of local tumour.
Any surgeon performing a chest wall resection should do so with the intent of removing all tumour tissue to obtain the best prognosis for the patient. A well-planned, expert, first surgical attempt will have the greatest chance of success (obtaining clean histological margins). Dirty margins increase the risk of recurrence or metastasis significantly (Pirkey-Ehrhart et al 1995).
Chest wall reconstruction may be needed if more than three or four ribs are removed, and methods of achieving this include advancement of the diaphragm, latissimus dorsi flap and synthetic mesh or combinations (Matthieson et al 1992). Rib replacement with artificial ribs or grafting of contralateral autogenous ribs has been reported (Duan et al 2006, Tunçözgür et al 1999).
In general, the long-term prognosis for dogs with chest wall tumours is somewhat dependent on histological type. OSA has the poorest prognosis (Chapter 21) because of the high rate of metastasis, with MST of between 12 and 17 weeks for tumours treated with surgery alone (Baines et al 2002, Matthieson et al 1992). CSA and fibrosarcomas (FSA) had significantly longer MST due to the lower rates of metastasis, with MST for CSA ranging from 10 to 36 months (Baines et al 2002, Matthieson et al 1992, Pirkey-Ehrhart et al 1995). MST for FSA has been reported to range from 17 to 84 weeks with surgery alone (Baines et al 2002, Pirkey-Ehrhart et al 1995). Rib haemangiosarcoma (HSA), as expected, has a poor prognosis due to early metastatic spread (MST 90 days) (Pirkey-Ehrhart et al 1995).
LUNG TUMOURS
Primary lung tumours in dogs
These are uncommon tumours with a reported incidence of 4 cases per 100 000. Prevalence is increasing, however, which may be due either to increased longevity as primary lung tumours are typically seen in older dogs (median age is 10 years) or to increased exposure to secondary smoking. There appears to be a no particular breed or sex predilection.
The most common histological type seen is adenocarcinoma (bronchial, bronchoalveolar, alveolar), which comprises about 80% of primary lung tumours (Ogilvie et al 1989). Squamous cell carcinoma (SCC) is the second most common. Others include chondromas, sarcomas, adenomas, fibromas and plasmacytomas. Nodular lung disease (abscess, cyst, granuloma (e.g. fungal disease), parasite migration, heartworm disease) and canine pulmonary lymphomatoid granulomatosis (very rare) are differential diagnoses; lung lobe torsions may radiographically appear tumour-like.
Primary lung tumours in cats
Lung tumours in cats are also rare and again are typically seen in older cats; diagnosis also appears to be on the increase. Typically in cats the caudal lung fields appear to be most frequently affected (Hahn & McEntee 1997). Most primary lung tumours in cats are SCC, and 75% of feline primary lung tumours metastasize (Hahn & McEntee 1997) (Figure 14.5). In addition to local lymph nodes and other lung fields, metastasis may occur to multiple digits, and swollen toes and lameness may be the presenting clinical sign rather than respiratory signs. Amputation is not palliative and the prognosis for these patients is poor (Gottfried et al 2000, van der Linde-Sipman & van den Ingh 2000).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree