Chapter 3. Congenital, developmental, or hereditary abnormalities in animals
CHAPTER CONTENTS
Objectives29
General principles of ocular embryology in relation to spontaneous developmental ocular diseases30
Abnormalities associated with infectious diseases or maternal intoxication32
Abnormalities associated with specific animal breeds34
Collie eye anomaly (CEA) 34
Merle and white spot coat color (merle ocular dysgenesis) 34
Congenital ocular anomalies in Rocky Mountain horses 34
Persistent hyperplastic primary vitreous (PHPV) and persistent hyperplastic tunica vasculosa lentis (PHTVL) 37
Retinal dysplasias 39
The vitreoretinopathies – vitreoretinal dysplasia 39
Oculo-skeletal dysplasia syndrome 41
Goniodysgenesis and other anterior segment dysgenesis syndromes 45
Peter’s anomaly and persistent pupillary membranes 46
Congenital failure in the formation of the anterior segment, anterior segment dysplasia or dysgenesis 47
Iris coloboma 49
Scleral coloboma 49
Congenital corneal edema in association with multifocal defects in Descemet’s membrane and endothelium 49
OBJECTIVES
• To illustrate the pathological changes seen in developmental ocular malformations, in a way that is helpful to both the diagnostic pathologist and the clinician who seeks insight into the pathology and pathogenesis of clinically observed lesions
• These cases are infrequently seen in a pathology collection. Of case submissions to the COPLOW collection, 2% are congenital disease or abnormalities of ocular development. Typically, only cases in which the abnormality leads to euthanasia or enucleation are submitted. Examples include:
▪ Euthanasia because of other concurrent developmental problems
▪ Euthanasia because the affected animal may be considered unsuitable for breeding
▪ Euthanasia or enucleation because of disfiguring eye disease
▪ Enucleation because of glaucoma
• Almost every species and breed of domestic animal has a list of genetic or presumed inherited diseases which are seen in greater numbers in the specific breeds. However, few of these conditions are regularly sampled for histopathology, and, in general, only those which are represented in the COPLOW collection are presented in this chapter.
GENERAL PRINCIPLES OF OCULAR EMBRYOLOGY IN RELATION TO SPONTANEOUS DEVELOPMENTAL OCULAR DISEASES (Figure 3.1, Figure 3.2, Figure 3.3 and Figure 3.4)
• The optic vesicle bulges out from the primitive neural tube ectoderm, making contact with the surface ectoderm, thus stimulating the local ectoderm to form the lens placode (Fig. 3.1)
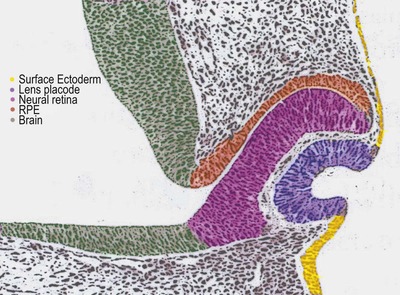 |
| Figure 3.1 Schematic drawing of early embryogenesis of ocular tissues. The lens placode forms and then invaginates in response to the optic vesicle making contact with surface ectoderm. With the subsequent invagination of the optic vesicle to form the double-layered optic cup. (Adapted with permission from Inagaki S, Kotani T 2002 Examination of the rat eye at the early stage of development with osmium tetroxide staining.) |
• Lens placode invaginates to form the lens vesicle which separates from the surface ectoderm (Fig. 3.2)
▪ Incomplete separation of the lens vesicle leads to developmental abnormalities of the anterior segment, involving the lens, cornea and anterior uvea.
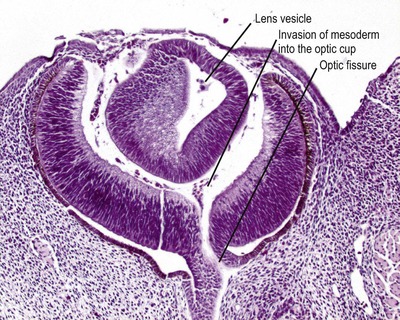 |
| Figure 3.2 Early embryonic eye. Photomicrograph of an embryonic goat eye showing the lens vesicle separated from the surface ectoderm and early stage retinal differentiation. The section passes through the optic fissure, through which mesenchyme enters the developing globe to form the earliest vitreous. |
• Invagination of the optic vesicle to form a bi-layered optic cup
▪ Failure of normal growth and invagination of the optic vesicle may lead to optic cyst, anophthalmia, microphthalmia, or combined abnormalities of the brain and eye.
• Invasion of vessels and associated mesenchyme into the optic cup to form the primary vitreous and tunica vasculosa lentis
▪ Failure of the primary vitreous and tunica vasculosa lentis to form can lead to microphthalmia
• Sprouting of the neuroepithelium from the anterior margin of the optic cup to form the double-layered ciliary and iridal epithelia (Fig. 3.3)
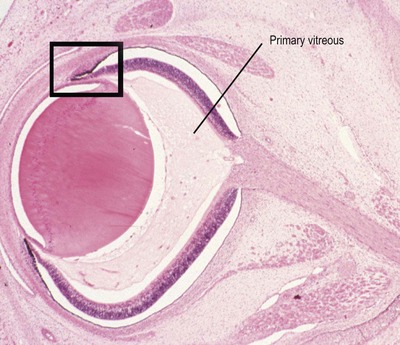 |
| Figure 3.3 Late embryonic eye. Photomicrograph of a bovine embryonic eye showing the primary vitreous, and the forward budding of neural tissue at the margins of the optic cup to initiate development of the ciliary body and iris (box). |
• Closure of the optic fissure separating the vasculature of the primary vitreous from the mesenchyme outside the optic cup, and establishment of a continuous inner neuroretina and an outer retinal pigment epithelium .
▪ Failure of optic fissure closure is one mechanism that can lead to the formation of typical scleral colobomata, usually in the dependant posterior globe
▪ Colobomatous microphthalmos may result from subsequent failure to establish the intraocular pressure that normally contributes to globe expansion
• Development of a multi-layered neuroretina, extension of retinal ganglion cell axons into the optic nerve, formation of ciliary and iridal epithelia, and the formation of lens fibers and eventually sutures (Fig. 3.4).
▪ Failures in the normal development of the retinal and/or uveal neuroepithelia, and lens fibers may be responsible for retinal dysplasia, optic nerve aplasia/hypoplasia, uveal colobomas and congenital cataract, respectively.
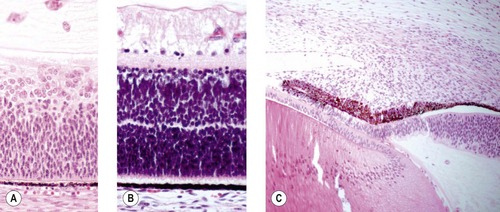 |
| Figure 3.4 Progressive differentiation of the tissues of the optic cup. (A) Photomicrograph of early retinal development before clear differentiation of distinct layers. (B) Photomicrograph showing the development of and separation of the layers of the retina. (C) Photomicrograph showing continued development of the ciliary body and iris by molding and proliferation of mesenchymal tissue. |
• Establishment and remodeling of the stromal layers of the eye including the uveal, scleral, and corneal stroma
▪ Failure of this normal mesenchymal development may be responsible for goniodysgenesis, choroidal hypoplasia, persistent pupillary membrane, Peter’s anomaly, variations in uveal pigmentation, and some colobomatous lesions
• Regression of the hyaloid vasculature of the primary vitreous and tunica vasculosa lentis and formation of the secondary vitreous (Fig. 3.5)
▪ Abnormalities in this process are associated with persistent hyperplastic primary vitreous, persistent hyperplastic tunica vasculosa lentis, and persistent hyaloid artery, as well as vitreoretinopathies such as vitreoretinal dysplasia.
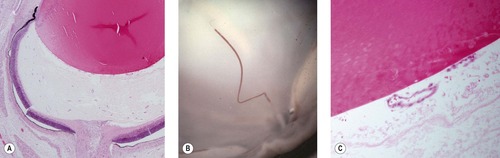 |
| Figure 3.5 Differentiation of the vitreous. Vascular structures in the primary vitreous undergo regression as the primary vitreous is replaced by the avascular secondary vitreous. (A) Subgross photomicrograph showing blood vessels in the primary vitreous. (B) Magnified gross photograph of a neonatal bovine eye showing a vestige of the hyaloid artery. (C) Photomicrograph showing vestiges of the tunica vascularis lentis. |
• Vascularization of the retina, and the continued development of retinal ganglion cells and their axons, which enter the optic nerve (Fig. 3.6)
▪ Failure of these processes leads to neovascular sprouting into the vitreous and optic nerve hypoplasia, respectively.
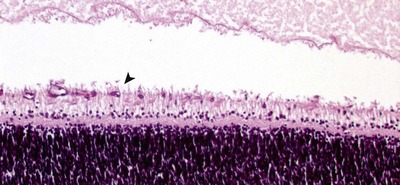 |
| Figure 3.6 Retinal blood vessel growth. Photomicrograph of a neonatal canine retina showing retinal blood vessel proliferation and differentiation. The arrow points to a newly formed vessel in the developing nerve fiber layer. |
• The full development of a multi-layered retina with functional and differentiated photoreceptors (this process continues after birth) (Fig. 3.7)
▪ Failure results in retinal dysplasia or photoreceptor dystrophies.
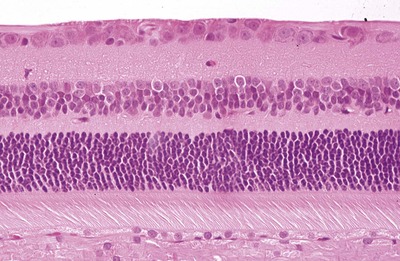 |
| Figure 3.7 The fully-differentiated feline retina in the area centralis. Photomicrograph of the normal, fully-differentiated feline retina sampled in the area centralis, the region with the highest density ganglion cells. |
• Many of the ocular tissues continue to develop throughout the early post-natal period, or, as in the case of the lens, throughout life. Abnormalities of these processes can lead to diseases that are not manifest in young animals, e.g. many forms of inherited cataract, hence they will not be covered in this chapter.
Comparative Comments
These general principles of ocular embryology apply to humans as well as other vertebrates. Specific congenital abnormalities in the human eye will be considered in the chapters which follow, discussing specific tissues within the eye and in the orbit and adnexa.
The various congenital anomalies arise because of variation in size, location, organization, or amount of tissue that represents a departure from normal.
A number of congenital abnormalities in humans fall under the classification of hamartomas or choristomas.
• A hamartoma is an excessive amount of mature tissue (hypertrophy and/or hyperplasia) occurring in a location in which that tissue is usually found. An example of a hamartoma would be any of the hereditary phakomatoses such as neurofibroma (a mass of mature neural tissue and fibroblasts) occurring in the orbit. No such collection of congenital disorders is described in animals
• A choristoma, in contrast, consists of normal, mature tissue in an abnormal location. Embryologically, this is a result of one or two germ layers forming mature tissue that is not normally found in that topographic location. An example of a choristoma is lens occurring in the lid (a phakomatous choristoma).
ABNORMALITIES ASSOCIATED WITH INFECTIOUS DISEASES OR MATERNAL INTOXICATION
Cyclopia or, more correctly, synophthalmos, in lambs, Veratrum californicum toxicity (Fig. 3.8)
• This is caused by consumption of alkaloids from the weed Veratrum californicum (Western False Hellebore) by the pregnant ewe on day 14 of gestation
• The 14 day embryo is undergoing gastrulation to form a neural tube with lateral symmetry
• Affected lambs have numerous developmental anomalies of the face, including arhinencephaly characterized by a proboscis-like nose developed above the fused globes
• Arhinencephaly with synophthalmos in humans is associated with very similar facial and ocular anomalies but no single teratogenic association has been made.
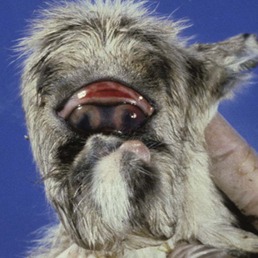 |
| Figure 3.8 Veratrum californicum induced teratogenesis. A neonatal lamb has toxic synophthalmos, caused by maternal ingestion of Veratrum californicum on day 14 of gestation. (Courtesy of Ron Riis, Diplomate – American College of Veterinary Ophthalmology). |
Abnormal ocular development in cattle associated with maternal infection with bovine viral diarrhea – mucosal disease (BVD-MD) virus (Figure 3.9 and Figure 3.10)
• Caused by a pestivirus
• In post-natal infection, the virus causes a necrotizing epitheliotropic disease characterized by diarrhea
• Infection of the fetus is associated with several syndromes
▪ Early maternal infections (before 40 days’ gestation) results in infertility, or fetal death and resorption
▪ Infections later may be associated with abortion
▪ Infection after 40 days gestation can result in normal, immune-deficient, or stunted calves that are immune-tolerant of the virus and remain persistent carriers of the virus. These calves are of great importance in the spread and control of the disease
▪ Some calves infected after 40 days’ gestation develop congenital abnormalities (Fig. 3.9)
– Cerebellar hypoplasia ‘dummy calf’
– Brachygnathia
– Ocular abnormalities, typically seen in calves infected later than 76 days but before 150 days gestation, include:
○ Microphthalmos
○ Microphakia/cataract
○ Retinal detachment
○ Retinal atrophy/dysplasia
○ Spindle cell metaplasia of the retinal pigment epithelium
○ Immunohistochemistry for BVD-MD virus antigen shows staining in blood vessels and punctate staining in the outer plexiform layer (Fig. 3.10).
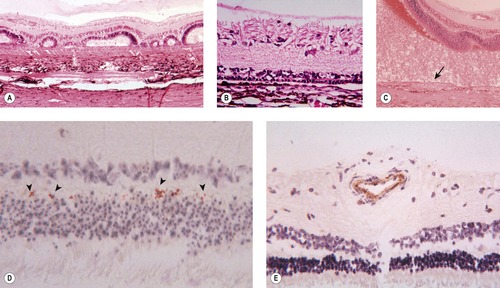 |
| Figure 3.10 Retinal changes seen in congenital BVD infection. (A) Dysplastic retinal development. (B) Outer retinal (photoreceptor) hypoplasia. (C) Redundant spindle cell tissue proliferates from the metaplastic retinal pigment epithelium (RPE) (arrow). (D) BVD immunohistochemistry labels foci in the inner plexiform layer (arrowheads). (E) BVD immunohistochemistry labels blood vessels. |
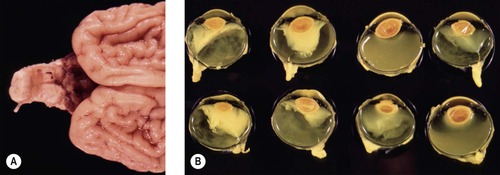 |
| Figure 3.9 Teratogenic effects of maternal infection with bovine virus diarrhea (BVD). (A) A neonatal calf brain showing profound hypoplasia or atrophy of the cerebellum. (B) Gross photograph showing both eyes from four affected calves with microphthalmos, protein exudation and lens displacement. |
Retinal dysplasia associated with perinatal infections in dogs and cats
• In cats infected in utero with feline panleukopenia virus retinal dysplasia is frequently associated with cerebellar hypoplasia
• Retinal necrosis and dysplasia have been reported following experimental infection with feline leukemia virus
• Dogs surviving neonatal canine herpesvirus infection demonstrate retinal dysplasia, necrosis and degeneration, often associated with other ocular abnormalities related to panuveitis.
Comparative Comments
The principle infectious embryopathies in humans are congenital rubella syndrome (Gregg’s syndrome) consisting of cataracts, cardiovascular defects, mental retardation, and deafness; cytomegalic inclusion disease; congenital syphilis; and toxoplasmosis.
The major drug embryopathies associated with a variety of eye changes in humans are: fetal alcohol syndrome, maternal thalidomide ingestion during the first trimester of pregnancy, and lysergic acid diethylamide (LSD) ingestion during the first trimester of pregnancy.
ABNORMALITIES ASSOCIATED WITH SPECIFIC ANIMAL BREEDS
Collie eye anomaly (CEA) (Fig. 3.11)
• Although a common disorder in dogs, specimens are seldom submitted for histopathological evaluation. Only six cases are logged into the COPLOW collection
• A congenital, recessively inherited, ocular disease seen in Collies and Shetland Sheepdogs, and also in Australian Shepherds, Border Collies, Lancashire Heelers and other herding breeds. The genetics of the disorder are discussed in greater detail in Chapter 11
• Bilateral but not always symmetrical
• A wide spectrum of severity of phenotypic expression is recognized, including:
▪ All cases demonstrate a regionally defined area of choroidal hypoplasia and segmental tapetal aplasia lateral to the optic disc. In isolation, this lesion has no clinically appreciable effect on vision
▪ Tortuous retinal blood vessels, may be apparent, most obvious in the area of choroidal hypoplasia
▪ Segmental lamina cribrosa or scleral defect (coloboma) with outward bulging or ectasia
– Although usually adjacent to or involving the lamina cribrosa, the scleral coloboma can be slightly separate from the disc
– Often unilateral
– Atrophic or dysplastic neural tissue, rather than uvea, lines the staphyloma
▪ Retinal detachment
– Can be localized to the area of the optic disc, i.e. peripapillary, or complete detachment.
– Usually unilateral
– Usually associated with a large coloboma
– Vitreous within the sub-retinal space probably gains access through tears in the atrophic neural tissue within the coloboma
▪ Retinal dysplasia
– Solitary or multifocal folds, or disorganized development of retinal layers with dysplasia may be seen
– Many authors and clinicians make a point of distinguishing retinal dysplasia, which has a component of disorganization, from retinal folds, which are simply a fold or wrinkle affecting all layers
▪ Hemorrhage into the posterior segment
– The source of hemorrhage may be neovascular sprouts from the choroid; the margins of the coloboma; the dysplastic retinal foci, or traction on existing retinal vessels, however, the source is usually undetermined
▪ Pre-iridal fibrovascular membranes (PIFVM)
– This secondary abnormality may, in turn, lead to peripheral anterior synechia followed by secondary neovascular glaucoma.
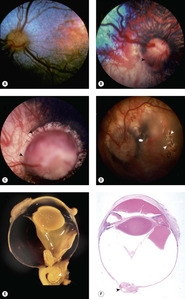 |
| Figure 3.11 Collie eye anomaly (CEA). (A–D) Fundus photographs show various stages of Collie eye anomaly. (A) Border Collie, 3 months old: choroidal hypoplasia is present temporally in this left eye. (B) Shetland Sheepdog, 7 weeks old: this right eye has choroidal hypoplasia (below arrow) and a peripapillary coloboma at the arrow. (C) Collie, 2 years old: a large coloboma (arrow) totally surrounds the optic disc. (D) Collie, 1 year old: the retina is detached in the left eye of this subalbinotic atapetal dog. The white arrows point to a hole in the detached retina. (E) Bouin’s fixed globe showing coloboma (arrow) near the optic nerve with retinal tissue entrapped within the coloboma. (F) Subgross photomicrograph of affected dog showing intraocular hemorrhage and optic nerve head coloboma (arrow). |
Merle and white spot coat color (merle ocular dysgenesis) (Fig. 3.12)
• As with CEA, merle ocular dysgenesis is seldom submitted to the pathology laboratory, although the condition is relatively common in certain breeds, such as the Australian Shepherd, specimens are rarely submitted for evaluation. There are only three cases in the COPLOW collection
• The merle gene is a color dilution gene that lightens the coat color. Many breeds or mixed breed dogs have merle coat color variants
• A dog homozygous for the merle gene will have a lighter coat color than a heterozygous genotype. Merle-to-merle breedings are not recommended
• The merle gene does not affect white coat color or white spots
• The merle gene does affect the color of the eye, although associated abnormalities may affect eyes with brown as well as blue irides
• Dogs of any breed, or mixed breeds, with the merle color dilution (homozygous merle) and abundant white spots are at risk of congenital abnormalities in ocular development. These abnormalities include:
▪ Microphthalmos
▪ Iridal abnormalities
– Iridal coloboma, iris hypoplasia
– Asymmetric pupil size, shape, or position (dyscoria and/or corectopia)
– Persistent pupillary membranes
▪ Lens abnormalities
– Microphakia
– Cataract
– Abnormal lens shape (coloboma)
– Lens luxation/subluxation
▪ Scleral defects (coloboma / staphyloma)
– Colobomata are usually not located at the optic disc as in CEA, and are frequently equatorial in location
– Colobomata may be very large (coloboma with cyst). The clinical picture in coloboma with cyst might be that of a fluid filled cyst making it difficult to recognize other ocular structures
▪ Retinal defects
– Retinal detachment, associated with severe dysplasia or large colobomata/staphylomas
– Retinal dysplasia, characterized by inner retinal thickening and the formation of inner retinal rosettes
• Affected dogs are frequently also deaf.
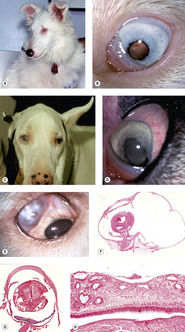 |
| Figure 3.12 Merle ocular dysgenesis. (A) Shetland Sheepdog, 8 months old: this color dilute, deaf dog is a result of a merle-X-merle breeding. (B) The left eye of the dog in (A) shows a microphthalmic globe with multiple ocular defects including a cataract and corectopia. (C) Great Dane, 6 years old: a harlequin-X-harlequin breeding resulted in this visual dog, which had dyscoria and corectopia in both microphthalmic eyes. (D) The left eye of the dog in (C) shows the dyscoria and hypermature cataract. (E) Clinical photograph showing a sharply delineated scleral coloboma. (F) Subgross photomicrograph of the same globe as (E) showing a large segmental scleral outpouching, retinal dysplasia and microcornea. (G) Subgross photomicrograph of a microphthalmic globe with retinal dysplasia. (H) Photomicrograph of the retina from (G) showing the typical features of retinal dysplasia in merle- and white-coated dogs. There is retinal thickening and jumbled differentiation. |
Congenital ocular anomalies in Rocky Mountain horses (Fig. 3.13)
• An inherited complex of multiple ocular abnormalities has been reported in the Rocky Mountain horse and related breeds. In this dominantly inherited disorder, that may have incomplete penetrance, disease expression is linked to the silver dapple locus, responsible for the chocolate coat color with white or flaxen mane and tail
• Bilateral ocular involvement is seen in affected horses
• Heterozygous horses have large, translucent, temporal ciliary cysts and may also have retinal dysplasia
• Homozygous horses have a complex of abnormalities that may include the following:
▪ Microphthalmos
▪ Cornea globosa
– In some affected horses the radius of curvature of the cornea appears to be shortened, leading to excessive anterior corneal curvature and the appearance of protruding eyes. These horses have abnormally deep anterior chambers
▪ Ciliary cysts
▪ Nuclear cataracts
▪ Lens subluxation.
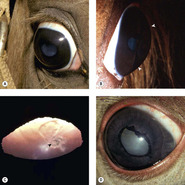 |
| Figure 3.13 Rocky Mountain horse. (A) The right globe has a megalocornea and a diffuse cortical cataract. (B) A lateral view illustrates the increased corneal curvature (arrows) in addition to the enlarged cornea, i.e. cornea globosa. (C) A close-up photograph showing the posterior cortical opacity (arrow) and dyscoria. (D) There is dyscoria and ectropion uvea. Diffuse lens opacity can be seen through the non-mobile pupil. |
Persistent hyperplastic primary vitreous (PHPV) and persistent hyperplastic tunica vasculosa lentis (PHTVL) (Fig. 3.14)
• There is a sporadic occurrence in any breed, but PHPV/PHTVL is considered to be inherited in the Doberman Pinscher and Staffordshire Bull terrier breeds.
• Specimens rarely submitted for evaluation
▪ There are only 12 cases logged into the COPLOW collection
– Three of the 12 are in Doberman Pinschers
• The primary vitreous, including the hyaloid vasculature and the tunica vasculosa lentis, provide a blood supply to the fetal lens, and in dogs completely regress shortly after birth
• In dogs with PHPV/PHTVL, the first observable abnormalities are seen about halfway through gestation (30–33 days), when the hyaloid vasculature and tunica vasculosa lentis appear over-developed relative to normal, and a retro-lental membrane may first be recognized
▪ The abnormalities are usually centered on the vitreous but there can be variable involvement of the whole retro-lental vascular system, and anterior tunica vasculosa lentis
▪ In moderate and severe cases, tissue may be recognized that would not be identified in the normal primary vitreous
– Cartilage
– Pigmented tissue
– Nests of glial tissue
▪ Posterior lenticonus, cataract and posterior lens capsule defect is often seen
▪ Intralenticular hemorrhage is sometimes seen
– Although hemorrhage within the lens is a good morphologic marker for a congenital abnormality of the fetal hyaloid vasculature, it does not discriminate between PHPV, PHTVL, and persistent hyaloid artery
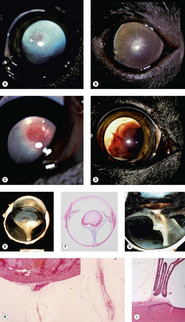 |
| Figure 3.14 Persistent hyperplastic primary vitreous and persistent hyperplastic tunica vasculosa lentis (PHPV/PHTVL). (A) Labrador Retriever, 1.5 years old: the arrow points to the network of fine blood vessels on the posterior lens capsule. A posterior axial cataract resulted from the PHPV. (B) Dachshund, 7 months old: fine vessels can be seen extending to the equator of the posterior lens in this bilateral condition. (C) Doberman Pinscher, 6 months old: the PHTVL resulted in an axial vascular area surrounded by a retrolental fibrovascular plaque (arrow). (D) German Pinscher, 1.5 years old: through the dilated pupil, the severe bleeding into the lens can be seen with PHPV. (E) Gross photograph showing PHPV and retinal detachment. (F) Subgross photomicrograph of the same globe as (E). (G) Gross photograph of retinal detachment and PHPV. (H) Photomicrograph showing a PHPV on the posterior pole of the lens (the insert is a higher magnification of the vessel in the mid-vitreous). (I) Photomicrograph of vascular structures extending from the pars plicata to the lens capsule. |
Retinal dysplasias (Fig. 3.15)
• An important hereditary abnormality in dogs, retinal dysplasia, is seldom encountered as a genetic disease in other species
• Globes with retinal dysplasia in isolation are almost never submitted to the diagnostic ocular pathology laboratory
▪ The only cases identified in the COPLOW collection are those in which retinal dysplasia accompanies other abnormalities of serious consequence to ocular health and/or vision
▪ Because so few cases are seen in a pathology collection, and because the only cases seen are in a special context, a diagnostic ocular pathologist has little beyond generalities to add to the understanding of these developmental disorders
• Definition: the disorganized development of retinal tissue
▪ Solitary or multi-focal disease
• Many breeds of dog are affected, and the reader is referred to the most recent edition of Ocular Disorders Presumed To Be Inherited in Purebred Dogs, with regular updates available from the Canine Eye Registration Foundation (CERF)
• Retinal dysplasia is characterized funduscopically as well-demarcated, linear, curvilinear or larger, geographic foci, that may be raised or wrinkled, and may be accompanied by pigment disturbance or evidence of associated retinal degeneration. In some cases, funduscopic lesions may not be readily identifiable until secondary degenerative changes become apparent
▪ Linear lesions are generally considered to represent ‘folds’, which some consider as a distinct entity not qualified to be considered as dysplasia, in the absence of significant tissue disorganization
– Some feel that simple retinal folds in puppies are likely to disappear with the subsequent maturation and physical enlargement of the globe
▪ Geographic lesions are likely to include retinal lesions beyond just folding
– Rosettes
– Thickening or thinning
– Jumbling of the retinal layers
– Focal retinal detachment, or local changes in the RPE, with accompanying retinal degeneration.
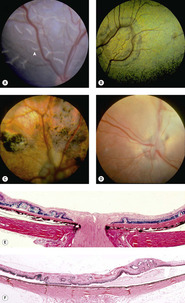 |
| Figure 3.15 Retinal dysplasia. (A) Cavalier King Charles Spaniel, 8 weeks old: multiple linear retinal folds (arrow) are seen in this immature fundus. (B) Golden Retriever, 2 years old: the large geographic tapetal lesion is discernible due to the change in tapetal coloration. (C) English Springer Spaniel, 18 months old: large areas of abnormal pigmentation and tapetal hyperreflectivity are indicative of severe geographic retinal dysplasia. (D) English Springer Spaniel, 2.5 years old: a complete retinal detachment obscures the fine detail of the underlying tapetum and non-tapetal pigmented fundus. (E,F) Photomicrographs show multifocal retinal dysplasia, characterized by retinal folds and disorganization of the retinal layers. |
The vitreoretinopathies – vitreoretinal dysplasia (Figure 3.16 and Figure 3.17
By virtue of the blinding effect of retinal detachment, intraocular hemorrhage and the risk of neovascular glaucoma, vitreoretinopathy is more often seen in the pathology laboratory than retinal dysplasia alone, with 58 cases logged into the COPLOW collection.
• Congenital, inherited retinal detachment/non-attachment is recognized in Bedlington and Sealyham terriers. For a comprehensive breakdown of the breeds affected by vitreoretinal dysplasia, the reader is referred to the most recent edition of Ocular Disorders Presumed To Be Inherited in Purebred Dogs, with regular updates available from the Canine Eye Registration Foundation (CERF)
• This diagnosis is made when there is abnormal development of both the vitreous and the retina. Because the two tissues are so intimately connected, disease of one impacts the health of the other
• The vitreous body is formed by scant spindle cells, a connection to the retina by attachment to the inner limiting membrane (a basal lamina secreted by retinal Müller cells), and hyaluronic acid secreted by the ciliary epithelium
• The vitreous changes seen include:
Get Clinical Tree app for offline access

▪ Areas of liquid vitreous which can only be recognized on gross examination, prior to embedding
▪ Areas of more dense, organized, or ‘solid’ vitreous




– These areas create traction on the retina and can be associated with subsequent retinal detachment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


